Abstract
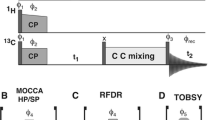
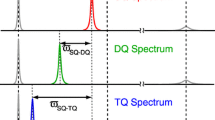
13CHD2 methyl isotopomers are particularly useful to study methyl dynamics in proteins because, as compared with other methyl isotopomers, the 13C relaxation mechanism for this isotopomer is straightforward. However, in the case of proteins, where (ωτ)2 ≫ 1, the refocused INEPT pulse sequence does not completely suppress unwanted 13CH3 signals. The presence of weak 13CH3 peaks is usually not a serious problem for smaller proteins because there are relatively few methyl signals and they are sharp; however, signal overlap becomes more common as the size of the protein increases. We overcome this problem by preparing a protein using a 98% D2O cell culture medium containing 3-13C pyruvic acid, 50–60% deuterated at the 3-position, and 4-13C 2-ketobutyric acid, 98% and 62% deuterated at the 3- and 4-positions, respectively. This approach significantly reduces the population of the CH3 isotopomer while optimizing the production of 13CHD2, the isotopomer desired for 13C relaxation measurements. In larger proteins where the deuterium T2 may be too short to measure accurately, we also suggest the alternative measurement of the proton T2 of the 13CH2D methyl isotopomer, because these protons are well-isolated from other protons in these highly deuterated samples.
Similar content being viewed by others
References
Gardner, K.H. and Kay, L.E. (1997) J. Am. Chem. Soc., 119, 7599–7600.
Goto, N.K., Gardner, K.H., Mueller, G.A., Willis, R.C. and Kay, L.E. (1999) J. Biomol. NMR, 13, 369–374.
Ishima, R., Louis, J.M. and Torchia, D.A. (1999) J. Am. Chem. Soc., 121, 11589–11590.
Ishima, R., Louis, J.M. and Torchia, D.A. (2001a) J. Mol. Biol., 305, 515–521.
Ishima, R., Petkova, A.P., Louis, J.M. and Torchia, D.A. (2001b) J. Am. Chem. Soc., 123, 6164–6171.
Kay, L.E., Bull, T.E., Nicholson, L.K., Griesinger, C., Schwalbe, H., Bax, A. and Torchia, D.A. (1992) J. Magn. Reson., 100, 538–558.
Lee, A.L., Urbauer, J.L. and Wand, A.J. (1997) J. Biomol. NMR, 9, 437–440.
LeMaster, D.M. (1999) J. Am. Chem. Soc., 121, 1726–1742.
LeMaster, D.M. and Kushlan, D.M. (1996) J. Am. Chem. Soc., 118, 9255–9264.
Louis, J.M., Clore, G.M. and Gronenborn, A.M. (1999) Nat. Struct. Biol., 6, 868–875.
Mahalingam, B., Louis, J.M., Reed, C.C., Adomat, J.M., Krouse, J., Wang, Y.F., Harrison, R.W. and Weber, I.T. (1999) Eur. J. Biochem., 263, 238–245.
Mueller, G.A., Choy, W.Y., Yang, D., Forman-Kay, J.D., Venters, R.A. and Kay, L.E. (2000) J. Mol. Biol., 300, 197–212.
Muhandiram, D.R., Yamazaki, T., Sykes, B.D. and Kay, L.E. (1995) J. Am. Chem. Soc., 117, 11536–11544.
Rosen, M.K., Gardner, K.H., Willis, R.C., Parris, W.E., Pawson, T. and Kay, L.E. (1996) J. Mol. Biol., 263, 627–636.
Sorensen, O.W. and Ernst, R.R. (1983) J. Magn. Reson., 51, 477–489.
Weber, I.T., Wu, J., Adomat, J., Harrison, R.W., Kimmel, A.R., Wondrak, E.M. and Louis, J.M. (1997) Eur. J. Biochem., 249, 523–530.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ishima, R., Louis, J.M. & Torchia, D.A. Optimized labeling of 13CHD2 methyl isotopomers in perdeuterated proteins: Potential advantages for 13C relaxation studies of methyl dynamics of larger proteins. J Biomol NMR 21, 167–171 (2001). https://doi.org/10.1023/A:1012482426306
Issue Date:
DOI: https://doi.org/10.1023/A:1012482426306