Abstract
Purpose. The purpose of this research was to examine a targeted prodrug strategy to increase the absorption of a poorly water-soluble lipophilic compound.
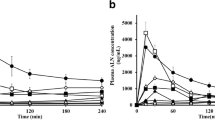
Methods. Three water-soluble prodrugs of Cam-4451 were synthesized. The amino acid (Cam-4562, Cam-4580) or phosphate (Cam-5223) ester prodrugs introduced moieties ionized at physiological pH and targeted intestinal brush-border membrane enzymes for reconversion to the parent. Selectivity for reconversion of the three prodrugs was examined in rat intestinal perfusate and brush-border membrane suspensions. Bioavailability of Cam-4451 in rats was evaluated after administering orally as the parent or as prodrugs in a cosolvent vehicle or in methylcellulose.
Results. Cam-5223 was highly selective for reconversion at the brush-border, but was rapidly reconverted in intestinal perfusate. Cam-4562 was not as selective but was more stable in the perfusate, whereas Cam-4580 was neither selective nor stable. Oral bioavailability of Cam-4451 was 14% after dosing as the parent in the cosolvent vehicle, 39% and 46%, respectively, as Cam-4562 and Cam-5223. Oral bioavailability was only 3.6% when the parent was dosed in methylcellulose, whereas the bioavailability was 7-fold higher when dosed as the phosphate prodrug.
Conclusions. Water-soluble prodrugs that target brush-border membrane enzymes for reconversion can be useful in improving drug oral bioavailability.
Similar content being viewed by others
REFERENCES
C. A. Lipinski, F. Lombardo, B. W. Dominy, and P. J. Feeney. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Del. Rev. 23:3–25 (1997).
G. L. Amidon, G. D. Leesman, and R. L. Elliott. Improving intestinal absorption of water-insoluble compounds: a membrane metabolism strategy. J. Pharm. Sci. 69:1363–1368 (1980).
W. D. Stein. The molecular basis of diffusion across cell membranes. In W. D. Stein (ed.), The Movement of Molecules across Cell Membranes, Academic Press, New York, 1967, pp. 65–125.
O. H. Chan, H. L. Schmid, B. S. Kuo, D. S. Wright, W. Howson, and B. H. Stewart. Absorption of Cam-2445, an NK1 neurokinin receptor antagonist: in vivo, in situ, and in vitro evaluations. J. Pharm. Sci. 85:253–257 (1996).
B. H. Stewart, O. H. Chan, R. H. Lu, E. L. Reyner, H. L. Schmid, H. W. Hamilton, B. A. Steinbaugh, and M. D. Taylor. Comparison of intestinal permeabilities determined in multiple in vitro and in situ models: relationship to absorption in humans. Pharm. Res. 12:693–699 (1995).
A. Tsuji, T. Terasaki, I. Tamai, and H. Hirooka. H+ gradient-dependent and carrier-mediated transport of cefixime, a new cephalosporin antibiotic, across brush-border membrane vesicles from rat small intestine. J. Pharmacol. Exp. Ther. 241:594–601 (1987).
G. L. Amidon, J. Kou, R. L. Elliott, and E. N, Lightfoot. Analysis of models for determining intestinal wall permeabilities. J. Pharm. Sci. 69:1369–1373 (1980).
D. Fleisher, R. Bong, and B. H. Stewart. Improved oral drug delivery: solubility limitations overcome by the use of prodrugs. Adv. Drug Del. Rev. 19:115–130 (1996).
C. N. TenHoor, and B. H. Stewart. Reconversion of fosphenytoin in the presence of intestinal alkaline phosphatase. Pharm. Res. 12:1806–1809 (1995).
H. Boxenbaum. Interspecies variation in liver weight, hepatic blood flow, and antipyrine clearance: extrapolation of data to benzodiazepines and phenytoin. J. Pharmacokinet. Biopharm. 8:165–176 (1980).
B. Davies and T. Morris. Physiological parameters in laboratory animals and humans. Pharm. Res. 10:1093–1095 (1993).
V. J. Stella, W. N. A. Charman, and V. H. Naringrekar. Prodrugs: Do they have advantages in clinical practice? Drugs. 29:455–473 (1985).
G. L. Amidon. Drug derivatization as a means of solubilization: Physicochemical and biochemical strategies. In S. H. Yalkowsky (ed.), Techniques of Solubilization of Drugs, Marcel Dekker, New York, 1981, pp. 183–211.
B. H. Stewart and M. D. Taylor. Prodrug approaches for improving peptidomimetic drug absorption. In M. D. Taylor and G. L. Amidon (eds.), Peptide-Based Drug Design: Controlling Transport and Metabolism, American Chemical Society, Washington, D.C., 1995, pp. 199–217.
D. Fleisher, K. C. Johnson, B. H. Stewart, and G. L. Amidon. Oral absorption of 21-corticosteroid esters: a function of aqueous stability and intestinal enzymes activity and distribution. J. Pharm. Sci. 75:934–939 (1986).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chan, O.H., Schmid, H.L., Stilgenbauer, L.A. et al. Evaluation of a Targeted Prodrug Strategy to Enhance Oral Absorption of Poorly Water-Soluble Compounds. Pharm Res 15, 1012–1018 (1998). https://doi.org/10.1023/A:1011969808907
Issue Date:
DOI: https://doi.org/10.1023/A:1011969808907