Abstract
Purpose. This study determines comparative bioavailability of diclofenac sodium lotion compared to an aqueous solution after topical application to viable human skin in vitro. In addition, the difference between a single dose and multiple doses (8 times) was also determined.
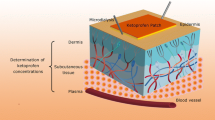
Methods. An in vitro flow-through diffusion cell system was employed, using radiolabelled diclofenac sodium.
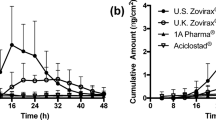
Results. Multiple doses of lotion (2 μl/cm2 and 5 μl/cm2) delivered a total of 40.1 ± 17.6 μg and 85.6 ± 41.4 μg diclofenac, respectively, at 48 h, compared to only 9.4 ± 2.9 μg and 35.7 ± 19.0 μg absorbed after topical application of diclofenac as an aqueous solution (P < 0.05). A single dose study showed no statistical difference between diclofenac delivered in lotion or an aqueous solution. Over 48 h the total absorption from lotion was 10.2 ± 6.7 μg and 26.2 ± 17.6 μg (2 μl/cm2 and 5 μl/cm2, respectively), compared to 8.3 ± 1.5 μg and 12.5 ± 5.7 μg from an aqueous solution. Both single doses of lotion and aqueous diclofenac showed decreased diclofenac absorption into the receptor fluid between 12 and 24 h. However, when applied multiple times, absorption from lotion was continually increasing up to 48 h. The total dose accountability ranged from 76.8 ± 8.2% to 110.6 ± 15.1% of the applied dose.
Conclusions. Diclofenac lotion exhibited enhanced diclofenac percutaneous absorption rate through human skin (mass, flux and partition coefficient) when applied a multiple number of times and this enhanced absorption was maintained over 48 h. This suggests that a constituent of the lotion (DMSO) will enhance human skin absorption of diclofenac when used in a multi-dose regimen, but not after a single dose.
Similar content being viewed by others
REFERENCES
B. L. Seth. Comparative pharmacokinetics and bioavailability study of percutaneous absorption of diclofenac from two topical formulations containing drug as a solution gel and as an emulsion gel. Arzneim-Forsch. 42:120–122 (1992).
H.-O. Ho, F.-C. Huang, T. D. Sokoloski, and M.-T. Shue. The influence of cosolvents on the in vitro percutaneous penetration of diclofenac sodium from a gel system. J. Pharm. Pharmacol. 46:636–642 (1994).
A. Sioufi, F. Pommier, F. Boschet, J. Godbillion, D. Lauoignat, and D. Salliere. Percutaneous absorption of diclofenac in healthy volunteers after single and repeated topical application of diclofenac emulgel. Biopharmaceut. Drug Disp. 15:441–449 (1992).
S. P. Vyas, R. Singh, and R. K. Asati. Liposomally encapsulated diclofenac for sonophoresis induced systemic delivery. J. Microencapsulation. 12:149–154 (1995).
T. Nishihata, K. Kotera, Y. Nakano, and M. Yamamazaki. Rat percutaneous transport of diclofenac and influence of hydrogenated soya lecitin. Chem. Pharm. Bull. 35:3807–3812 (1987).
Y. Obata, K. Takayama, H. Okabe, and T. Nagai. Effect of cyclic monoterpenes on percutaneous absorption in the case of a water-soluble drug (diclofenac sodium). Drug Des. Del. 6:319–328 (1991).
T. Nishihata, A. Kamada, K. Takahashi, K. Matsumoto, K. Shinozaki, Y. Tabata, M. Keigami, T. Miyagi, and N. Tastumi. Percutaneous absorption of diclofenac in rats and humans: Aqueous gel formulation. Int. J. Pharm. 46:1–7 (1988).
L. N. Hurst, D. H. Brown, and K. A. Murray. Prolonged life and improved quality for stored skin grafts. Plas. Reconstr. Surg. 73:105–109 (1984).
R. L. Bronaugh, R. F. Stewart, and J. E. Storm. Extent of cutaneous matabolism during percutaneous absorption of xenobiotics. Toxicol. Appl. Pharmacol. 99:534–543 (1989).
R. C. Wester, J. Christoffel, T. Hartway, N. Poblete, and H. I. Maibach. Cadaver human skin viability for in vitro percutaneous absorption: Storage and detrimental effects of heat-separation and freezing. Pharm. Res. 15:82–84 (1998).
R. C. Wester, P. K. Noonan, and H. I. Maibach. Variations in percutaneous absorption of testosterone in the rhesus monkey due to anatomic site of application and frequency of appliation. Arch. Dermatol. Res. 116:186–188 (1980).
R. C. Wester and H. I. Maibach. Cutaneous pharmacokinetics: 10 steps to percutaneous absorption. Drug Met. Rev. 14:169–205 (1983).
R. C. Wester, J. Melendres, L. Sedik, and H. I. Maibach. Percutaneous absorption of azone following single and multiple doses to human volunteers. J. Pharm. Sci. 83:124–125 (1994).
J. Radermacher, D. Jentsch, M. A. Scholl, T. Lustinetz, and J. C. Frolich. Diclofenac concentrations in synovial fluid and plasma after cutaneous application in inflammatory and degenerative joint disease. Br. J. Clin. Pharmacol. 31:537–541 (1991).
D. A. W. Bucks, H. I. Maibach, and R. H. Guy. Percutaneous absorption of steroids: Effect of repeated application. J. Pharm. Sci. 74:1337–1339 (1985).
R. C. Wester, P. K. Noonan, and H. I. Maibach. Percutaneous absorption of hydrocortisone increases with long-term administration: In vivo studies in the rhesus monkey. Arch. Dermatol. 116:186–188 (1980).
M. Muller, H. Mascher, C. Kikuta, S. Schafer, M. Brunner, G. Domer, and H. G. Eichler. Diclofenac concentrations in defined tissue layers after topical administration. Clin. Pharmacol. Ther. 62:293–299 (1997).
R. B. Stoughton. Dimethylsulfoxide induction of a steroid reservoir in human skin. Arch. Dermatol. 91: 657–660 (1965).
W. W. Shen, A. G. Danti, and F. N. Bruscato. Effect of nonionic surfactants on percutaneous absorption of salicylic acid and sodium salicylate in the presence of dimethylsulfoxide. J. Pharm. Sci. 65: 1780–1783 (1976).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hewitt, P.G., Poblete, N., Wester, R.C. et al. In Vitro Cutaneous Disposition of a Topical Diclofenac Lotion in Human Skin: Effect of a Multi-Dose Regimen. Pharm Res 15, 988–992 (1998). https://doi.org/10.1023/A:1011961607089
Issue Date:
DOI: https://doi.org/10.1023/A:1011961607089