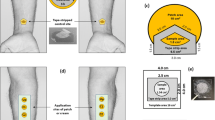
Abstract
Biopharmaceutical assessment of topical drug formulations is widely carried out by using vertical diffusion cells (Franz type cell). Although Franz diffusion cell model is well designed for percutaneous absorption studies, the extent of drug penetration within the skin requires more adapted device. Recently, we have developed a new patented versatile, easy-to-use, and disposable diffusion cell called VitroPharma. In this study we have assessed the cutaneous bioavailability of caffeine as hydrophilic compound model using Franz diffusion cell and VitroPharma. The percutaneous absorption of caffeine assessed with Franz diffusion cell andVitroPharmawas characterized by using (i) finite dose model and (ii) classical pharmacokinetic analysis. Furthermore, the follow-up of caffeine penetration within the skin was determined by sequential measurements of tissular drug concentration throughout the time of skin exposure with VitroPharma. However, classical experimental design using Franz diffusion cell involved unique determination of tissular concentration at the final point of skin exposure protocol. Finally, device equivalence between Franz diffusion cell and VitroPharma was claimed from percutaneous absorption data analysis. Concomitant assessment of dual penetration and permeation kinetics by using VitroPharma reinforced the understanding of skin drug delivery.
Similar content being viewed by others
References
Schneider M, Windbergs M, Daum N, et al. Crossing biological barriers for advanced drug delivery. Eur J Pharm Biopharm 2013; 84: 239–41.
Naik A, Kalia YN, Guy RH. Transdermal drug delivery: overcoming the skin’s barrier function. Pharm Sci Technol To 2000; 3: 318–26.
Potts RO, Guy RH. Predicting skin permeability. Pharm Res 1992; 9: 663–9.
Salmon D, Kassai B, Roussel L, et al. Ex vivo absorption of promestriene from oil-in-water emulsion into infant foreskin. Int J Pharm 2013; 456: 121–4.
Williams FM. In vitro studies-how good are they at replacing in vivo studies for measurement of skin absorption? Environ Toxicol Pharmacol 2006; 21: 199–203.
Hartung T. From alternative methods to a new toxicology. Eur J Pharm Biopharm 2010; 77: 338–49.
EURL-ECVAM, Method summaries- Percutaneous Absorption Review Document. Available at: http://ecvam-dbalm.jrc.ec. europa.eu/, consulted on the 10 January 2015.
Kansy M, Senner F, Gubernator K. Physicochemical high throughput screening: parallel artificial membrane permeation assay in the description of passive absorption processes. J Med Chem 1998; 41: 1007–10.
Gardner CR, Almarsson O, Chen H, et al. Application of high throughput technologies to drug substance and drug product development. Comput Chem Eng 2004; 28: 943–53.
OCDE, Guidelines for the testing of chemicals- n°428, Skin Absorption: in vitro Method. 2004.
Prow TW, Grice JE, Lin LL, et al. Nanoparticles and microparticles for skin drug delivery. Adv Drug Deliv Rev 2011; 63: 470–91.
Wagner H, Kostka KH, Lehr CM, Schaefer UF. Drug distribution in human skin using two different in vitro test systems: comparison with in vivo data. Pharm Res 2000; 17: 1475–81.
Mah CS, Kochhar JS, Ong PS, Kang L. A miniaturized flowthrough cell to evaluate skin permeation of endoxifen. Int J Pharm 2013; 441: 433–40.
Levintova Y, Plakogiannis FM, Bellantone RA. An improved in vitro method for measuring skin permeability that controls excess hydration of skin using modified Franz diffusion cells. Int J Pharm 2011; 419: 96–106.
Salmon D, Pirot F, Rodriguez L, et al. Device for studying the permeability of artificial, synthetic or biological membranes. Patent WO2013057401, priority date: 10 october 2011.
Scheuplein RJ. Mechanism of percutaneous absorption. II. Transient diffusion and the relative importance of various routes of skin penetration. J Invest Dermatol 1967; 48: 79–88.
Roberts MS, Anissimov YG, Gonsalvez RA. Mathematical Models in Percutaneous Absorption. In: Bronaugh RL, Maibach HI, Marcel Dekker, eds. Percutaneous Absorption, Drugs–Cosmetics–Mechanisms–Methology. 3rd ed. 1999: 3–55.
Anissimov YG, Jepps OG, Dancik Y, Roberts MS. Mathematical and pharmacokinetic modelling of epidermal and dermal transport processes. Adv Drug Deliv Rev 2013; 65: 169–90.
Dias M, Farinha A, Faustino E, Hadgraft J, Pais J, Toscano C. Topical delivery of caffeine from some commercial formulations. Int J Pharm 1999; 182: 41–7.
Selzer D, Hahn T, Naegel A, et al. Finite dose skin mass balance including the lateral part: Comparison between experiment, pharmacokinetic modeling and diffusion models. J Control Release 2013; 165: 119–28.
Gee CM, Nicolazzo JA, Watkinson AC, Finnin BC. Assessment of the lateral diffusion and penetration of topically applied drugs in humans using a novel concentric tape stripping design. Pharm Res 2012; 29: 2035–46.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Salmon, D., Gilbert, E., Gioia, B. et al. New easy handling and sampling device for bioavailability screening of topical formulations. Eur J Dermatol 25 (Suppl 1), 23–29 (2015). https://doi.org/10.1684/ejd.2015.2551
Published:
Issue Date:
DOI: https://doi.org/10.1684/ejd.2015.2551