Abstract
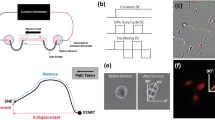
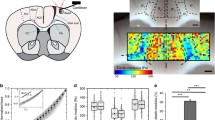
We have studied the cellular basis for recovery from acute spinal cord injury induced by applied electric fields. We have emphasized this recovery is due to the regeneration of spinal axons around and through the lesion, and have begun to evaluate the contribution of other cells to the recovery process. We have imposed a voltage gradient of about 320 μV/mm across puncture wounds to the adult rat spinal cord in order to study the accumulation and orientation of GFAP+ astrocytes within and adjacent to the lesion. This electric field was imposed by a miniaturized electronic implant designed to alternate the polarity of the field every 15 minutes. Astrocytes are known to undergo hyperplastic transformation within injured mammalian cords forming a major component of the scar that forms in response to injury. We have made three observations using a new computer based morphometry technique: First, we note a slight shift in the orientation of astrocytes parallel to the long axis of the spinal cord towards an imaginary reference perpendicular to this axis by approximately 10°—but only in undamaged white matter near the lesion. Second, the relative number of astrocytes was markedly, and statistically significantly, reduced within electrically—treated spinal cords, particularly in the lesion. Third, the imposed voltage gradient statistically reduced the numbers of astrocytes possessing oriented cell processes within the injury site compared to adjacent undamaged regions of spinal cord.
Similar content being viewed by others
References
Anderson, M. J., Waxman, S. G. & Laufer, M. (1983) Fine structure of regenerated ependyma and spinal cord in stenarchus alfifrons. Anatomical Record 205, 73–83.
Bernstein, J. J. & Bernstein, M. E. (1967) Effect of glialependymal scar and teflon arrest on the regenerative capacity of goldfish spinal cord. Experimental Neurology 19, 25–32.
Bernstein, J. J. & Bernstein, M. E. (1969) Ultrastructure of normal regeneration and loss of regenerative capacity following teflon blockage in goldfish spinal cord. Experimental Neurology 24, 538–557.
Blight, A. R. (1985) Delayed demyelination and macrophage invasion: A candidate for secondary cell damage in spinal cord injury. Central Nervous System Trauma 2, 299–315.
Blight, A. R. (1992) Macrophages and inflammatory damage in spinal cord injury. Journal of Neurotrauma 9, s83–s91.
Blight, A. R., McGinnis, M. E. & Borgens, R. B. (1990) Cutaneus trunci muscle reflex of the guinea pig. Journal of Comparative Neurology 296, 614–633.
Borgens, R. B. (1982) What is the role of naturally produced electric current in vertebrate regeneration and healing? International Review of Cytology 76, 245–298.
Borgens, R. B. (1992) Applied voltages in spinal cord reconstruction: History, strategies, and behavioral models. In Spinal Cord Dysfunction, Volume III: Functional Stimulation (edited by Illis, L. S.) pp. 110–145. Oxford: Oxford Medical Publications.
Borgens, R. B. (1999) Electrically mediated regeneration and guidance of adult mammalian spinal axons into polymeric channels. Neuroscience 91, 251–264.
Borgens, R. B. & Bohnert, D. M. (1997) The responses of mammalian spinal axons to an applied DC voltage gradient. Experimental Neurology 145, 376–389.
Borgens, R. B. & McCaig, C. D. (1989) Artificially controlling axonal regeneration and development by applied electric fields. In: Electric Fields in Vertebrate Repair. Coauthored by R. B. Borgens, K. R. Robinson, J.W. Vanable, Jr., and M. E. McGinnis (edited by Liss, A. L. New York) chap. 4. pp. 117–170.
Borgens, R. B., Roederer, E. & Cohen, M. J. (1981) Enhanced spinal cordregeneration in lamprey by applied electric fields. Science 213, 611–617.
Borgens, R. B., Blight, A. R., Murphy, D. J. & Stewart, L. (1986) Transected dorsal column axons within the guinea pig spinal cord regenerate in the presence of an applied electric field. Journal of Comparative Neurology 250, 168–180.
Borgens, R. B., Blight, A. R. & McGinnis, M. E. (1987) Behavioral recovery induced by applied electric fields after spinal cord hemisection in guinea pig. Science 238, 366–369.
Borgens, R. B., Blight, A. R. & McGinnis, M. E. (1990) Functional recovery after spinal cord hemisection in guinea pigs: The effects of applied electric fields. Journal of Comparative Neurology 296, 634–653.
Borgens, R. B., Toombs, J. P., Blight, A. R., McGinnis, M. E., Bauer, M. S., Widmer, W. R. & Cook, J. R. (1993a) Effects of applied electric fields on clinical cases of complete paraplegia in dogs. Restorative Neurology and Neuroscience 5, 305–322.
Borgens, R. B., Metcalf, M. E. & Blight, A. R. (1993b) Delayed application of direct current electric fields in experimental spinal cord injuries. Restorative Neurology and Neuroscience 5, 173–179.
Borgens, R. B., Shi, R., Mohr, T. J. & Jaeger, C. B. (1994) Mammalian cortical astrocytes align themselves in a physiological voltage gradient. Experimental Neurology 128, 41–49.
Borgens, R. B., Toombs, J. P., Breur, G., Widmer, W. R., Water, D., Harbath, A. M., March, P. & Adams, L. G. (1999) An imposed oscillating electrical field improves the recovery of function in neurologically complete paraplegic dogs. Journal of Neurotrauma 16, 639–657.
Carlson, S. L., Parrish, M. E., Springer, J. E., Doty, K. & Dossett, L. (1999) Acute inflammatory response in spinal cord following impact injury. Experimental Neurology 151, 77–88.
Chernoff, E. A. G. & Stocum, D. L. (1995) Developmental aspects of spinal cord and limb regeneration. Development, Growth and Differentiation 37, 133–147.
Clemente, C. D. (1955) Structural regeneration in the mammalian central nervous system and the role of neuroglia and connective tissue. In Regeneration in the Central Nervous System (edited by Windle, W. F.) pp. 147–161. Springfield, IL: Thomas.
Clemente, C. D. (1964) Regeneration in the vertebrate central nervous system. International Review of Biology 6, 257–301.
Coggeshall, R. E. & Lekan, H. A. (1996) Methods for determining numbers of cells and synapses: A case for more uniform standards of review. The Journal of Comparative Neurology 364, 6–15.
Davies, S. J. A., Goucher, D. R., Doller, C. & Silver, J. (1999) Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. Journal of Neuroscience 19, 5810–5822.
Fehlings, M. G., Tator, C. H. & Linden, R. D. (1988) The effect of direct-current field on recovery from experimental spinal cord injury. Journal of Neurosurgery 68, 781–792.
Freeman, L. W., MacDougall, J., Turbes, C. C. & Bowmann, D. E. (1960) The treatment of experimental lesions of the spinal cord of dogs with trypsin. Journal of Neurosurgery 17, 259–265.
Fujita, T., Yoshimine, T., Maruno, M. & Hayakawa, T. (1998) Cellular dynamics of macrophages and microglial cells in reaction to stab wounds in rat cerebral cortex. Acta Neurochir urgica 140, 275–279.
Geisert Jr., E. E., Yang, L. & Irwin, M. H. (1996) Astrocyte growth, reactivity, and the target of the antiproliferative antibody, TAPA. Journal of Neuroscience 16, 5478–5487.
Geisert Jr., E. E., Seo, H., Sullivan, C. D., Yang, L. J. & Grefe, A. (1998) A novel approach to identify proteins associated with the inhibition of neurite growth. Journal of Neuroscience Methods 79, 21–29.
Hatten, M. E., Liem, R. K. H., Shelanski, M. L. & Mason, C. A. (1991) Astroglia in CNS injury. Glia 4, 233–243.
Hinkle, L., McCaig, C. D. & Robinson, K. R. (1981) The direction of growth of differentiating neurons and myeloblasts from frog embryos in an applied electric field. Journal of Physiology 314, 121–135.
Jaffe, L. F. & Nuccitelli, R. (1977) Electrical controls of development. Annual Review of Biophysics and Bioengineering 6, 445–476.
Jaffe, L. F. & Poo, M. M. (1979) Neurites grow faster toward the cathode than the anode in a steady field. Journal of Experimental Zoology 209, 115–127.
Leskovar, A., Moriarty, L. J., Turek, J., Schoenlein, I. A. & Borgens, R. B. (2000) The macrophage in acute neural injury: Changes in cell numbers over time and levels of cytokine production in mammalian central and peripheral nervous systems. Journal of Experimental Biology 203, 1783–1795.
Levin, M. & Ernst, S. G. (1995) Applied AC and DC magnetic fields cause alterations in the mitotic cycle of early sea urchin embryos. Bioelectromagnetics 16, 231–240.
Levin, M. & Ernst, S. G. (1997) Applied DC magnetic fields cause alterations in the time and cell dicisions and developmental abnormalitites in early sea urchin embryos. Bioelectromagnetics 18, 255–263.
Liuzzi, F. J. & Lasek, R. J. (1987) Astrocytes block axonal regeneration in mammals by activating the physiological stop pathway. Science 237, 642–645.
McCaig, C. D. (1986) Dynamic aspects of amphibian neurite growth and the effects of an applied electric field. Journal of Physiology 375, 55–69.
McCaig, C. D. (1987) Spinal neurite reabsorption and regrowth in vitro depend on the polarity of an applied electric field. Development 100, 31–41.
Miranda, J. D., White, L. A., Marcillo, A. E., Willson, C. A., Jagid, J. & Whittemore (1999) Induction of Eph B3 after spinal cord injury. Experimental Neurology 156, 218–222.
Moriarty, L. J. & Borgens, R. B. (1999) The effect of an applied electrical field on macrophage accumulation within the subacute spinal injury. Restorative Neurology and Neuroscience 14, 53–64.
Moriarty, L. J., Duerstock, B. S., Bajaj, C. L., Lin, K. & Borgens, R. B. (1998) Two and three dimensional computer graphic evaluation of the subacute spinal cord injury. Journal of Neurological Science 155, 121–137.
Orida, N. & Feldman, J. D. (1982) Directional protrusive pseudopodial activity and motility in macrophages induced by extracellular electric fields. Cell Motility 2, 243–255.
Patel, N. & Poo, M. M. (1982) Orientation of neurite growth by extracellular electric fields. Journal of Neuroscience 2, 483–496.
Reier, P. J. & Houle, J. D. (1988) The glial scar: Its bearing on axonal elongation and transplantation approaches to CNS repair. In Advances in Neurology, Vol. 47: Functional Recovery in Neurological Diseases (edited by Waxman, S. G.) pp. 87–138. New York: Raven Press.
Reier, P. J., Stensaas, L. J. & Guth, L. (1983) Spinal cord reconstruction, in the astrocytic scar as an impediment to regeneration in the central nervous system (edited by Kao, C. C., Bunge, R. P. & Reier, R. J.) pp. 163–195. Springfield, IL: Charles C. Thomas.
Rudge, J. S. & Silver, J. (1990) Inhibition of neurite outgrowth on astroglial scars in vitro. Journal of Neuroscience 10, 3594–3603.
Savio, T. & Schwab, M. E. (1989) Rat CNS white matter, but not gray matter, is nonpermissive for neuronal cell adhesion and fiber outgrowth. Journal of Neuroscience 9, 1126–1133.
Schwab, M. E., Kapfhammer, J. P. & Bandtlow, C. E. (1993) Inhibitors of neurite growth. Annual Review of Neuroscience 16, 565–595.
Simpson, S. B. (1983) Fasciculation and guidance of regenerating central axons by the ependyma. In Neuroelectric Research (edited by Kao, C. C., Bunge, R. P. & Reier, R. J.) pp. 151–161. Springfield, IL: Charles C. Thomas.
Singer, M., Norlander, R. H. & Eger, M. (1979). Axonal guidance during embryogenesis and regeneration in the spinal cord of the newt: The blueprint hypothesis of neuronal pathway patterning. Journal of Comparative. Neurology 185, 1–22.
Stensaas, L. J. (1983) Regeneration in the spinal cord of the newt, Notopthalmus (triturus) pyrrhogaster. In Spinal Cord Regeneration (edited Bunge, R. P. & Reier, P. J.) pp. 121–149. New York: Raven Press.
Strautman, A. F., Cook, R. J. & Robinson, K. R. (1990) The distribution of free calcium in transected spinal axons and it modulation by applied electrical fields. Journal of Neuroscience 10, 3564–3575.
Windle, W. F. (1956) Regeneration of axons in the vertebrate central nervous system. Physiological Reviews 36, 427–440.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moriarty, L.J., Borgens, R.B. An oscillating extracellular voltage gradient reduces the density and influences the orientation of astrocytes in injured mammalian spinal cord. J Neurocytol 30, 45–57 (2001). https://doi.org/10.1023/A:1011917424450
Issue Date:
DOI: https://doi.org/10.1023/A:1011917424450