Abstract
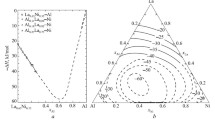
Three composition joins in the Na2O–B2O3–SiO2system at constant Na2O contents of 5, 10, and 15 mol % are studied by the high-temperature method of determining oxygen ion exponents pO for oxide melts. It is found that the basicity of melts increases in going from the binary sodium borate system to the sodium silicate system. The acid–base properties of ternary melts are simulated under the assumption that their basicity is determined by the interaction in pseudobinary systems. It is shown that the basicity of the studied melts is governed, to a large extent, by the formation of the Na2O · B2O3· 2SiO2ternary compound.
Similar content being viewed by others
REFERENCES
Konakov, V.G., Shakhmatkin, B.A., and Shultz, M.M., Determining the Relative Basicity of B2O3-SiO2, B2O3-GeO2, and GeO2-SiO2 Melts, Fiz. Khim. Stekla, 1994, vol. 20, no. 4, p. 443-448 [Glass Phys. Chem. (Engl. transl.), 1994, vol. 20, no. 4, pp. 298-301].
Konakov, V.G., A Study of the Oxygen Ion Activity in Sodium Silicate Melt, Fiz. Khim. Stekla, 1990, vol. 16, no. 5, pp. 753-758.
Konakov, V.G., A Study of the Relative Basicity of Melts in the BaO-B2O3 System, Fiz. Khim. Stekla, 1999, vol. 25, no. 1, p. 70-77 [Glass Phys. Chem. (Engl. transl.), 1999, vol. 25, no. 1, pp. 56-60].
Toropov, N.A., Barzakovskii, V.P., Lapin, V.V., et al., Diagrammy sostoyaniya silikatnykh sistem. Spravochnik. Vyp. 3. Troinye sistemy (A Handbook on State Diagrams of Silicate Systems: Ternary Systems), Leningrad: Nauka, 1972, issue 3.
Shultz, M.M., Kozhina, E.L., and Shakhmatkin, B.A., Influence of B2O3 and Al2O3 on the Chemical Potential of an Alkali Oxide in Silicate Melts, Fiz. Khim. Stekla, 1989, vol. 15, no. 3, pp. 500-507.
Asai, K. and Yokokawa, T., Thermodynamic Activity of Na2O in Na2O-B2O3-SiO2 Melt, Trans. Jpn. Inst. Met., 1982, vol. 23, no. 9, pp. 571-577.
Shakhmatkin, B.A. and Shultz, M.M., Thermodynamic Properties and Structure of Alkali Borate Melts, Fiz. Khim. Stekla, 1982, vol. 8, no. 3, pp. 270-276.
Appleman, D.E. and Clark, J.R., Crystal Structure of Reedmergnierite, a Boron Albite, and Its Relation to Feldspar Crystal Chemistry, Am. Mineral., 1965, vol. 50, nos. 11-12, pp. 1827-1850.
Polyakova, I.G., Tokareva, E.V., and Popova, T.V., On the Responsibility of “Boroleucite Anomaly” for Alkali Borosilicate Glass Properties, Proc. XVII Int. Congress on Glass, Beijing, 1995, vol. 2, pp. 381-386.
Yun, Y.H. and Bray, P.J., Nuclear Magnetic Resonance Studies of the Glasses in the System Na2O-B2O3-SiO2, J. Non-Cryst. Solids, 1978, vol. 27, no. 3, pp. 363-380.
Dell, W.J., Bray, P.J., and Xiao, S.Z., 11B NMR Studies and Structural Modeling of Na2O-B2O3-SiO2 Glasses of High Soda Content, J. Non-Cryst. Solids, 1983, vol. 58, no. 1, pp. 1-16.
Nieto, M.I. and Oteo, J.L., Structure des verres de borosilicate alcalins presentant une demixtion, Verres Refract., 1986, vol. 40, no. 1, pp. 19-31.
Konakov, V.G. and Shultz, M.M., Study of Relative Basicity (PO-Indexes) of the Systems Li2O-SiO2, K2O-SiO2, and Li2O-K2O-SiO2, Proc. XVIII Int. Congress on Glass, San Francisco, 1998, p. AB88.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Konakov, V.G., Kozhina, E.L. & Shultz, M.M. A Study of the Acid–Base Properties of Melts in the Na2O–B2O3–SiO2System: I. Composition Joins with a Na2O Content of Less Than 20 mol %. Glass Physics and Chemistry 27, 182–187 (2001). https://doi.org/10.1023/A:1011392729329
Issue Date:
DOI: https://doi.org/10.1023/A:1011392729329