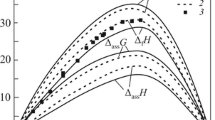
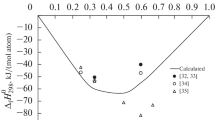
The mixing enthalpies of liquid ternary Al–La–Ni alloys are determined by isoperibol calorimetry for sections (Al0.42La0.58)1–xNix (0 < x < 0.27) at 1430 K and (La0.25Ni0.75)1–xAlx at 1770 K (0 < < xAl < 0.3) or 1800 K (0.3 < xAl < 0.39). The experimental mixing enthalpies and those calculated with the Redlich–Kister model are characterized by exothermic effects. The minimum mixing enthalpy of the melts is –54 kJ/mol for composition Al0.4La0.2Ni0.4.












Similar content being viewed by others
References
T. Godecke, W. Sun, R. Luck, and K. Lu, “Metastable Al–Nd–Ni and stable Al–La–Ni phase equilibria,” Z. Metallkd., 92, No. 7, 717–722 (2001).
A. K. Abramyan, “Study of interactions in the nickel-rich phases of the aluminum–lanthanum–nickel system,” VINITI, 3782, 212–214 (1979).
M. H. Mendelsohn, D. M. Gruen, and A. E. Dwight, “LaNi5–x Al x is a versatile alloy system for metal hydride application,” Nature, 269, 45–47 (1977).
H. Yang, J. Q. Wang, and Y. Li, “Glass formation and microstructure evolution in Al–Ni–RE (RE = La, Ce, Pr, Nd and misch metal) ternary systems,” Philos. Mag., 87, No. 27, 4211–4228 (2007).
F. Ye and K. Lu, “Crystallization kinetics of Al–La–Ni amorphous alloy,” J. Non-Cryst. Solids, 262, No. 1–3, 228–235 (2000).
P. Si, X. Bian, W. Li, et al., “Relationship between intermetallic compound formation and glass forming ability of Al–Ni–La alloy,” Phys. Lett. A, 319, Nos. 3–4, 424–429 (2003).
T. Abe, M. Shimono, K. Hashimoto, et al., “Phase separation and glass-forming abilities of ternary alloys,” Scripta Mater., 55, No. 5, 421–424 (2006).
H. Feufel, F. Schuller, J. Schmid, and F. Sommer, “Calorimetric study of ternary liquid Al–La–Ni alloys,” J. Alloys Compd., 257, 234–244 (1997).
F. Sommer, J. Schmid, and F. Schuller, “Temperature and concentration dependence of the enthalpy of formation of liquid Al–La–Ni alloys,” J. Non-Cryst. Solids, 205–207, 352–356 (1996).
V. S. Sudavtsova, V. A. Makara, and V. G. Kudin, Thermodynamics of Metallurgical and Welding Alloys [in Russian], Logos, Kyiv (2005), Part 3, p. 216.
N. V. Kotova, N. I. Usenko, L. A. Romanova, and V. S. Sudavtsova, “Mixing enthalpies of ternary Al–Ni–La alloys,” Zh. Fiz. Khim., 87, No. 9, 1466–1470 (2013).
A. Pasturel, C. Chatillon-Colinet, and A. Percheron-Guégan, “Thermodynamic properties of LaNi4M compounds and their related hydrides,” J. Less-Common Met., 84, 73–78 (1982).
P. G. Zielinski and H. Matyja, “Influence of liquid structure on glass forming tendency,” in: Rapidly Quenched Metals Sec. Int. Conf., MIT Press, Cambridge (1975), pp. 237–248.
Institute for Materials Research, Tohoku University: http://wwwdb1.imr.tohoku.ac .jp/java_applet/Amor_Ternary/Al–La–Ni.html.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Poroshkovaya Metallurgiya, Vol. 55, Nos. 9–10 (511), Vol. 55, Nos. 9–10 (511), pp. 124–134, 2016.
Rights and permissions
About this article
Cite this article
Kudin, V.G., Shevchenko, M.O., Ivanov, M.I. et al. Thermodynamic Properties of Al–La–Ni Melts. Powder Metall Met Ceram 55, 603–611 (2017). https://doi.org/10.1007/s11106-017-9845-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-017-9845-0