Abstract
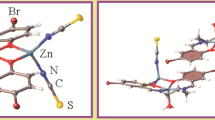
The complex [Zn(Ofpa)2(H2O)4] · 2H2O (Ofpa is 2-formylphenoxyacetate) is synthesized and characterized by X-ray structural analysis and IR spectroscopy. The crystal is monoclinic: a= 25.254(5), b= 6.952(1), c= 13.951(3) Å, β = 116.41(3)°, Z= 4, space group C2/c, R= 0.034. The zinc atom in the centrosymmetric complex is coordinated by two monodentate carboxylate ligands (Zn–O 2.123(1) Å) and by four water molecules (Zn–O 2.085(1) and 2.092(2) Å). The oxygen atom of the aldehyde group is not involved in coordination. Complexes and solvate water molecules in the crystal are united into a three-dimensional framework via hydrogen bonds and π–π interactions.
Similar content being viewed by others
REFERENCES
Smith, G., O'Reilly, E.J., Kennard, C.H.L., and White, A.H., J. Chem. Soc., Dalton Trans., 1985, no. 2, p. 243.
Kennard, C.H.L., O'Reilly, E.J., and Smith, G., Polyhedron, 1984, vol. 3, no. 4, p. 689.
Kennard, C.H.L., Stewart, S.W., O'Reilly, E.J., and Smith, G., Polyhedron, 1985, vol. 4, no. 4, p. 697.
Kennard, C.H.L., O'Reilly, E.J., Smith, G., and Mak, T.C.W., Aust. J. Chem., 1985, vol. 38, no. 9, p. 1381.
Kullbeg, L. and Choppin, G.R., Inorg. Chem., 1977, vol. 16, no. 11, p. 2926.
Haseqawa, Y. and Choppin, G.R., Inorg. Chem., 1977, vol. 16. no. 11, p. 2931.
Kerfoot, H.B. and Choppin, G.R., Inorg. Chem., 1979, vol. 18, no. 3, p. 787.
Smith, G., O'Reilly, E.J., and Kennard, C.H.L., Polyhedron, 1987, vol. 6, no. 5, p. 871.
Kennard, C.H.L., Smith, G., O'Reilly, E.J., and Mahohoran, P.T., Inorg. Chim. Acta, 1984, vol. 82, no. 1, p. 35.
Kennard, C.H.L., O'Reilly, E.J., Schiller, S., et al., Austr. J. Chem., 1986, vol. 39, no. 11, p. 1823.
Kennard, C.H.L., Smith, G., O'Reilly, E.J., et al., Inorg. Chim. Acta, 1982, vol. 59, no. 2, p. 241.
Shova, S., Novitski, G., Gdanets, M., et al., Abstracts of Papers, Natsional'naya kristallokhimicheskaya konferentsiya (National Crystal Chemistry Conf.), Chernogolovka, 1998, part 1, p. 229.
Forrester, A.R., Skilling, J., and Thomson, R.H., J. Chem. Soc., Dalton. Trans., 1974, no. 11, p. 2161.
Sheldrick, G.M., Acta Crystallogr., Sect. A: Found. Crystallogr., 1990, vol. 46, no. 6, p. 467.
Sheldrick, G.M., SHELXL93: Program for the Refinement of Crystal Structure, Göttingen: Univ. of Göttingen, 1993.
Korshunov, A.V., Primenenie molekulyarnoi spektroskopii v khimii (Application of Molecular Spectroscopy in Chemistry), Moscow: Nauka, 1966, pp. 159, 165, 200.
Elinkton, D.G., Primenenie spektroskopii v organicheskoi khimii, Moscow: Mir, 1967, p. 130.
Zolin, V.F., Kazanskaya, N.A., Moshinskaya, A.V., et al., Opt. Spektrosk., 1972, vol. 33, no. 5, p. 929.
Sechkarev, A.V. and Petrov, A.K., Opt. Spektrosk., 1965, vol. 19, no. 6, p. 904.
Chupakhina, R.A., Biryulina, V.N., Kasimova, L.V., et al., Zh. Obshch. Khim., 1986, vol. 56, no. 5, p. 1022.
Vashok, B. and Gramana, R., Indian J. Pure Appl. Phys., 1987, vol. 25, no. 5, p. 203.
Vashok, B. and Gramana, R., Indian J. Pure Appl. Phys., 1987, vol. 25, no. 5, p. 209.
Nakamoto, K., Infrared and Raman Spectra of Inorganic and Coordination Compounds, New York: Wiley, 1986. Translated under the title IK spektry i spektry KR neorganicheskikh i koordinatsionnykh soedinenii, Moscow: Mir, 1991.
Kharitonov, Yu.Ya. and Oleinik, I.I., Dokl. Akad. Nauk SSSR, 1987, vol. 294, no. 1, p. 151.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shova, S., Novitsky, G., Gdaniec, M. et al. Synthesis and Structure of Tetraaqua-bis(2-Formylphenoxyacetato)Zinc(II) Dihydrate. Russian Journal of Coordination Chemistry 27, 387–392 (2001). https://doi.org/10.1023/A:1011383607873
Issue Date:
DOI: https://doi.org/10.1023/A:1011383607873