Abstract
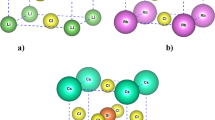
The ab initio local DFT method combined with the ab initio pseudopotential technique is used to calculate valence electron densities in series of crystal substances MA (M = Li, Na, K, Rb, Ag, Mg, Ca; A = F, Cl, Br, O, S) with a NaCl lattice. Systematic variations of valence electron density depending on the atomic number of anion and cation have been found. According to electron density distribution, the compounds are classified into three groups: a) oxides and fluorides; b) sulfides, chlorides, and bromides; c) noble metal halides. In oxides and fluorides, the maximum of valence electron density is in the middle of the M–M bond. In sulfides, chlorides, and bromides, the minimum density is in the middle of the M–M bond, with two symmetric maxima or two “shoulders” (depending on the atomic number of the cation) lying away from the center of the bond. In noble metal halides, the maximum of valence density is on the metal due to the presence of metal d-states, and the density map is rotated through 90° relative to the map of alkali and alkali earth metals, so that the Hal–Hal bond becomes an analog of the M–M bond.
Similar content being viewed by others
References
M. Bucher, Phys. Rev. B, 35, No. 6, 2923-2928 (1974).
G. K. Wertheim, J. E. Rowe, D. N. E. Buchanan, and P. H. Citrin, ibid., 51, No. 19, 13675-13680 (1995).
P. Jonnard, F. Vergand, C. Bonnelle, et al., ibid., 57, No. 19, 12111-12118 (1998).
J. M. Gillet and P. Cortona, ibid., 60, No. 12, 8569-8574 (1999).
A. Strachan, T. Cagin, and W. A. Goddard III, ibid., 60, No. 22, 15084-15093 (1999).
A. B. Gordienko and A. S. Poplavnoi, Phys. Status Solidi (b), 208, No. 1, 407-411 (1998).
S. Lundqvist and N. H. March (eds.), Theory of Inhomogeneous Electron Gas, Plenum, New York (1983).
G. H. Bachelet, D. R. Hamann, and M. Schlüter, Phys. Rev. B, 26, No. 8, 4199-4228 (1982).
Yu. N. Zhuravlev, Yu. M. Basalaev, and A. S. Poplavnoi, Izv. Vyssh. Uchebn. Zaved., Fiz. (1999); VINITI dep. No. 3772-B99.
A. A. Radtsig and V. M. Smirnov, Atomic and Atomic Ion Parameters [in Russian], Energoatomizdat, Moscow (1986).
V. P. Zhukov, Zh. Strukt. Khim., 38, No. 3, 554-583 (1997).
D. J. Chadi and M. L. Cohen, Phys. Rev. B, 8, No. 12, 5747-5753 (1973).
T. Penkala, Essays on Crystal Chemistry [Russian translation], Khimiya, TLeningrad (1974).
G. D. Mahan, Phys. Rev. B, 38, No. 11, 7841 (1988).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zhuravlev, Y.N., Basalaev, Y.M. & Poplavnoi, A.S. Electron Density Calculations for Crystals with a NaCl Lattice. Journal of Structural Chemistry 42, 172–176 (2001). https://doi.org/10.1023/A:1010434511684
Issue Date:
DOI: https://doi.org/10.1023/A:1010434511684