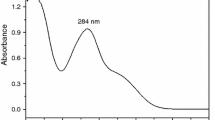
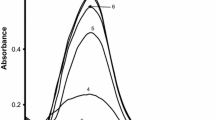
Abstract
Reaction between Ag2O and SO2 gives silver and Ag2SO4. Heating Ag2O/Ag2S mixtures comprising between 66.67 to 100.00 mol % of oxide, at forced flow of inert gas, gives only silver.
Similar content being viewed by others
REFERENCES
T. Suzuki: J. Phys. Soc. Jpn., 15, 2018 (1960)
J. Walczak, E. Tomaszewicz: Xlllth International Symposium on the Reactivity of Solids, 8-12 September, 1996, Hamburg, 9-PO-243.
S. Djurle: Acta Chem. Scand., 12, 1427 (1958).
R.C. Sharma, Y.A. Chang: Bull. Alloy Phase Diagrams, 7, 263 (1986).
H. Rau: J. Phys. Chem. Solids, 35, 1553 (1974).
J. Walczak, F. Boccuzzi, E. Lukaszczyk-Tomaszewicz: J. Alloy Compd., 224, 203 (1995).
Rights and permissions
About this article
Cite this article
Kurzawa, M., Tomaszewicz, E. Kinetics of Reactions between Some Compounds from the Three-Component Silver -Oxygen -Sulfur System. Reaction Kinetics and Catalysis Letters 70, 53–59 (2000). https://doi.org/10.1023/A:1010350329497
Issue Date:
DOI: https://doi.org/10.1023/A:1010350329497