Abstract
Arrhenius parameters values, in non-isothermal kinetic vaporisation processes for a series of compounds with related structures, have been calculated. This was made using a method of calculation that allows to find the most probable vaporisation mechanisms.
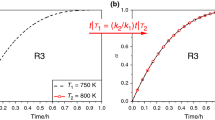
According to this method DTG curves were compared with some theoretical ones reported in literature, whose shape results to be only a function of the mechanisms. In this way the choice of the mathematical functions which can be inserted in the kinetic equations, was influenced by the shape of the DTG plots and other thermal analysis signals thus allowing to choose the most probable mechanisms.
The kinetic parameters derived from these mechanisms were compared, using statistical analysis, with those obtained from another method of calculation based on ‘a priori’ vaporisation mechanism chosen for the investigated liquid–gas transition.
The standard deviations of the slope and of the intercept, together with the standard deviation and the square correlation coefficient (r 2) of the linear regression equations related to the mechanisms of the two methods were calculated. Student t-test, Fisher F-test, confidence intervals (c.i.) and residuals valueswere also given.
Statistical analysis shows that the mechanisms obtained with the former method (diffusive and geometrical models) and the related Arrhenius parameters result to be more significant (in terms of probability) than the corresponding quantities of the latter for which a first-order model was chosen.
Similar content being viewed by others
References
J. Zsakó, J. Thermal Anal., 34 (1988) 1489.
J. Zsakó, J. Thermal Anal., 47 (1996) 1679.
J. Zsakó, J. Thermal Anal., 54 (1998) 921.
J. Zsakó, Cs. V·rhelyi and G. Liptay, Thermochim. Acta, 203 (1992) 297.
A. K. Galway and M. E. Brown, Thermochim. Acta, 300 (1997) 107.
A. K. Galway and M. E. Brown, Proc. R. Soc. London, A450 (1995) 501.
S. Clementi, F. Fringuelli, P. Linda and S. Savelli, Gazz. Chim. Ital., 105 (1975) 291.
W. H. Davis Jr. and W. H. Pryor, J. Chem. Educ., 53 (1976) 285.
S. Clementi, F. Fringuelli and S. Savelli, Chim. Ind. (Milan), 60 (1978) 598.
E. Tiley, Chem. Br., 21 (1985) 162.
O. Exner, Collect. Czech. Chem. Commun., 31 (1968) 3223.
J. Shorter, Correlation Analysis of Organic Reactivity, Wiley, New York, 1984.
F. Rodante, G. Catalani and M. Guidotti, J. Thermal Anal., 53 (1998) 937.
J. McCarty and M. Green, Instruction Manual for the Stanton Redcroft Simultaneous TG-DSC, January 1989.
M. J. Sanchez-Martin and M. Sanchez-Camazano, Thermochim. Acta, 126 (1988) 319.
D. Dollimore, Thermochim. Acta, 203 (1992) 7.
D. Dollimore, T. A. Evans, Y. F. Lee, G. P. Pee and F. W. Wilburn, Thermochim. Acta, 196 (1992) 255.
D. Dollimore, T. A. Evans, Y. F. Lee and F. W. Wilburn, Thermochim. Acta, 198 (1992) 249.
X. Gao, D. Chen and D. Dollimore, Thermochim. Acta, 223 (1993) 333.
V. Satava, Thermochim. Acta, 21 (1971) 423.
C. B. Doyle, J. Appl. Polym. Sci., 6 (1962) 639.
O. Vitali, Tavole Statistiche, Cacucci Editore, Bari 1994, p. 62.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rodante, F., Vecchio, S. Significance of Non-isothermal Kinetic Data. A statistical study. Journal of Thermal Analysis and Calorimetry 63, 433–455 (2000). https://doi.org/10.1023/A:1010196526966
Issue Date:
DOI: https://doi.org/10.1023/A:1010196526966