Abstract
A critical analysis of the use of an overall single rate reaction equation instead of the true rate equation corresponding to a complex process consisting in two consecutive reactions is presented. In accordance with this approximation, often used in the kinetic analysis of the system in which several reactions take place, the overall process is described by the apparent activation parameters (the apparent activation energy, E ap, and the apparent pre-exponential factor, A ap) and the apparent conversion function.
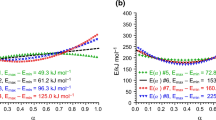
The theoretical isotherms (α=α(t), where a is the conversion degree and t is the time) have been simulated for a system in which two consecutive reactions occur. In this case, the apparent activation parameters depends on: (a) the considered range of the temperature; (b) the temperature, for a given conversion degree. It is shown that the apparent activation parameters are corrrelated by the compensation effect relationship: lnA ap=α*+β*E ap where α* and β* are the parameters of the linear regression.
The possibility of using the apparent kinetic parameters to predict the isotherms α=α(t) for temperatures lower than those for which these parameters were evaluated, is discussed.
Similar content being viewed by others
References
P. D. Garn, J. Thermal Anal., 7 (1975) 474.
A. K. Galwey, Adv. Catal., 26 (1977) 247.
P. K. Gallager and D. W. Johnson, Jr., Thermochim. Acta, 14 (1976) 255.
G. W. Collet and B. Rand, Thermochim. Acta, 41 (1980) 153.
J. D. Cooney, M. Day and D. M. Wiles, J. Appl. Polym. Sci., 220 (1984) 131.
H. Tanaka and N. Koga, J. Phys. Chem., 92 (1988) 7023.
P. K. Agrawal, J. Thermal Anal., 31 (1986) 73.
E. Segal and D. Fatu, Introduction to Non-isothermal Kinetics (in Romanian), Publishing House of the Romanian Academy, Bucharest 1983, Ch. VIII.
P. Budrugeac and E. Segal, Thermochim. Acta, 184 (1991) 25.
P. Budrugeac and E. Segal, Thermochim. Acta, 184 (1991) 33.
P. Budrugeac and E. Segal, J. Thermal Anal., 39 (1993) 1199.
P. Budrugeac and E. Segal, Thermochim. Acta, 221 (1993) 221.
P. Budrugeac and E. Segal, ICTAC.News, 28 (1995) 33.
P. Budrugeac, A. L. Petre and E. Segal, Thermochim. Acta, 275 (1996) 193.
J. Li, G. Zhang and J. Wang, Thermochim. Acta, 207 (1992) 219.
J. R. MacCallum and M. V. Murno, Thermochim. Acta, 203 (1992) 457.
S. Vyazovkin and W. Linert, Intern. Rev. Phys. Chem., 14 (1995) 355.
S. Vyazovkin and W. Linert, Chem. Phys., 193 (1995) 109.
S. Vyazovkin, Intern. J. Chem. Kinetics, 28 (1996) 95.
L. Audouin and J. Verdu, Polym. Degrad. Stabil., 31 (1991) 335.
V. Marcu and E. Segal, Thermochim. Acta, 35 (1980) 43.
H. Tanaka and N. Koga, J. Thermal Anal., 34 (1988) 685.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Budrugeac, P., Segal, E. On the Apparent Compensation Effect Found for Two Consecutive Reactions. Journal of Thermal Analysis and Calorimetry 62, 227–235 (2000). https://doi.org/10.1023/A:1010183316395
Issue Date:
DOI: https://doi.org/10.1023/A:1010183316395