Abstract
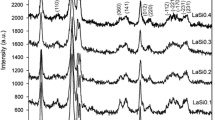
The compound obtained via state solid reaction of the La2O3 and SrO oxides and expose the room atmosphere shows the crystallographic data of the compound reported as La2SrOx. However, thermogravimetric, differential thermal analysis and XRD with controlled temperature indicated that the stoichiometry of the compound is 2La(OH)3-SrCO3, which structural parameters were determined by using the Rietveld method. It was verified that when the compound exposed at room atmosphere, the mixture oxide absorbs H2O and CO2 producing hydroxide and carbonate of lanthanum and strontium, respectively, which thermal decomposition occurs by the same steps, producing the La2O3-SrO.
Similar content being viewed by others
References
R. Doshi, Y. Shen and C. B. Alcock, Solid State Ionics, 68 (1994) 133.
Q. Sun, J. I. Di Cosimo, R. G. Herman, K. Klier and M. M. Bhasin, Catalysis Letters, 15 (1992) 371.
M. Xu and J. H. Lunsford, Catalysis Letters, 11 (1991) 295.
B. H. T. Chai and S. Mroczkowski, J. Crystal Growth, 44 (1978) 84.
K. Foger, M. Hoang and T. W. Turney, J. Mater. Sci., 27 (1992) 77.
H. Wakita, Bull. Chem. Soc. Japan, 51 (1978) 2879.
R. L. N. Sastry, S. R. Yoganarasimban, P. N. Mehrotra and C. N. R. Rao, J. Inorg. Nucl. Chem., 28 (1966) 1165.
S. A. Robbins, R. G. Rupard, B. J. Weddle, T. R. Maull md P. K. Gallagher, Thermochim. Acta, 269/270 (1995) 43.
E. L. Charsley, C. M. Earnest, P. K. Gallagher and M. I. Richardson, J. Thermal Anal., 40 (1993) 1415.
ICDD Diffraction Databases 1993–1994. Newtown Square: International Centre for Diffraction Data, 1988. PDF number 42-0343 (CD Rom).
D. R. Lide, Handbook of Chemistry and Physics, Boca Raton, Fbrida, 71st edition, 1990–1991, p. 4.
G. W. Beall, W. O. Milligan and H. A. Wolcott, J. Inorg. Nucl. Chem., 39 (1977) 65.
J. P. R. de Villiers, American Mineralogist, 56 (1971) 758.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Marques, R.F.C., Zorel, H.E., Crespi, M.S. et al. Thermal and Crystalographic Studies of Mixture La2O3-SrO Prepared Via Reaction in the Solid State. Journal of Thermal Analysis and Calorimetry 56, 143–149 (1999). https://doi.org/10.1023/A:1010160130461
Issue Date:
DOI: https://doi.org/10.1023/A:1010160130461