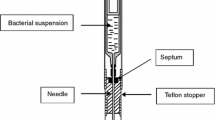
Abstract
This paper presents the basic equations of thermokinetics and the thermoanalytical curve equation for bacterial growth in conduction calorimeter on the basis of the basic theory of thermokinetics. The bacterial growths in the log phase for Vibro metschnikovii and Bacillus subtilis at different temperatures were calorimetrically investigated. The rate constant of bacterial growth, the cooling constant of the thermokinetic system, the generation time and the pre-exponential factor at different temperature were obtained, which allowed to evaluate the activation energy of bacterial growth (E a). According to the transition-state theory of chemical kinetics, the activation enthalpy (ΔS ≠), the activation Gibbs free energy (ΔG ≠) and equilibrium constant (K ≠) of the activated state at different temperatures were also obtained. The above results showed that the research method suggested in this paper was reasonable.
Similar content being viewed by others
References
Chang-Li Xie, Hou-Kuan Tang, Zhao-Hua and Song-Sheng Qu, Thermochim. Acta, 123 (1988) 33.
Hong-Lin Zhang, Hai-Tao Sun, Yong-Jun Liu, Qing-Zhu Shan and Xiu-Feng Sun, Thermochim. Acta, 223 (1993) 29.
P. Monk and I. Wadsö, Acta Chem. Scandinavic, 22 (1968) 1842.
L. Nunez Regueira, I. Gomez Orellane, N. Barros Pena, R. Chamy and J. M. Lema, Thermochim. Acta, 172 (1990) 163.
P. Weppen, J. Ebens, B. G. Muller and D. Schuller, Thermochim. Acta, 193 (1991) 135.
P. Backman, Thermochim. Acta, 172 (1990) 123.
E. H. Battley, Thermochim. Acta, 309 (1998) 17.
L. Marisonk, J. S. Liu, S. Ampuero, U. Von Stockar and B. Schenker, Thermochim. Acta, 309 (1998) 157.
E. Calvet and H. Prat, Recent Progress in Microcalorimetry, Pergamon Press, Oxfod 1963.
Yu Deng, Chem. J. Chinese Univ., 6 (1985) 621.
Xian-Cheng Zeng, Xiang-Guang Meng, Yuan-Qin Zhang and Min-Zu Chen, Chem. J. Chinese Univ., 18 (1997) 581.
Yong-Jun Liu, Yang-Jun Ding, Zhao-Dong Nan, Hai-Tao Sun and Hong-Lin Zhang, J. Thermal Anal., 50 (1997) 897.
W. J. Moore, Physical Chemistry, Longman Group Limited, London, 5th ed., 1972, p. 385.
I. Wadsö, Thermochim. Acta, 294 (1997) 1.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nan, ZD., Xiang, Y., Cheng, SQ. et al. Thermokinetic Research Method for Bacterial Growth in Conduction Calorimeter. Journal of Thermal Analysis and Calorimetry 63, 423–431 (2000). https://doi.org/10.1023/A:1010144510128
Issue Date:
DOI: https://doi.org/10.1023/A:1010144510128