Abstract
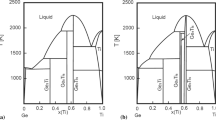
A semi-empirical model consistent with thermodynamical conditions of equilibrium was used and oriented to the calculation of phase diagrams of the binary systems H2 O-MgCl2 , H2 O-FeCl2 and H2 O-FeCl3 . For each solid phase, the exploitation of the experimental and bibliographical data gives a liquidus curve equation comprising a limited number of parameters. A such equation allows to calculate with precision the solubility of the stoichiometric solid phase in a large range of temperature and composition.
Similar content being viewed by others
Références
R. Cohen-Adad, M.-Th. Cohen-Adad, R. Ouän et F. Getzen, J. Chim. Phys., 87 (1990) 1441.
R. Cohen-Adad et M.-Th. Cohen-Adad, Entropie, 160 (1991) 47.
R. Cohen-Adad and J. W. Lorimer, I.U.P.A.C., Solubility Data-Series, Alcali Metal and Ammonium Chlorides in Water and D2O, Vol. 47, Pergamon Press, Oxford 1991.
R. Cohen-Adad, M.-Th. Cohen-Adad, D. Chehimi and A. Marrouche, Model for the Critical Evaluation of Solubility in Salt Systems, CODATA, Chambery 1994.
A. Atbir, L. Aneflous, M. El Hadek, R. Cohen-Adad et M.-Th. Cohen-Adad, Ann. Chim. Fr., Sciences des Matériaux, 21 (1996) 239.
A. Atbir, L. Aneflous, M. El Hadek, R. Cohen-Adad et M.-Th. Cohen-Adad, Ann. Chim. Fr., Sciences des Matériaux, 21 (1996) 347.
A. Atbir, A. Marrouche, M. El Hadek, R. Cohen-Adad and M.-Th. Cohen-Adad, J. Chim. Phys., 94 (1997) 1274.
A. Atbir, Thèse d.Etat Es-Sciences, Agadir, n°39/97, 1997.
A. Atbir, A. Marrouche, L. Aneflous, M. El Hadek, R. Cohen-Adad et M. Th. Cohen-Adad, J. Therm. Anal. Cal., (accepté, ref. JTAC 30-03/98), 1998.
W. F. Linke, Solubilities of Inorganic and Metal-Organic Compound, 4th ed., Vols 1 and 2; Amer. Chem. Soc., Van Nostrand, 1965.
O. V. Afonichkin and T. I. Gaidukova, Zh. Khim., 74 (1976) 30.
I. M. Chou and L. D. Phan, J. Chem. Eng. Data, 30 (1985) 216.
I. M. Chou and L. D. Phan, J. Chem. Eng. Data, 31 (1986) 145.
F. Schimmel, J. Amer. Chem. Soc., 54 (1952) 4689.
A. Atbir, S. Mançour Billah, A. Marrouche, M. El Hadek, R. Cohen-Adad et M. Th. Cohen-Adad, J. Therm. Anal. Cal., (accepté, ref. JTAC 41-09/98).
M. Mazghouni, R. Rokbani, M. Trabelsi, A. Sebaoun et N. Kbir-Ariguib, Bul. Soc. Chim., Fr., 7.8 (1981) 247.
K. H. Balarev and D. Spasov, Russ. J. Inorg. Chem., 25 (1980) 1551.
A. P. Shchedrina and L. I. Krasnova, Russ. J. Inorg. Chem., 14 (1969) 138.
A. P. Shchedrina, M. I. Ozerova and L. I. Krasnova, Russ. J. Inorg. Chem., 14 (1969) 1151.
A. P. Shchedrina, L. I. Krasnova and L. M. Mel.nichenko, Russ. J. Inorg. Chem., 15 (1970) 991.
A. P. Shchedrina and L. I. Krasnova, Russ. J. Inorg. Chem., 17 (1972) 122.
A. P. Shchedrina and L. M. Mel'nichenko, Russ. J. Inorg. Chem., 19 (1974) 897.
V. W. Voigt, Th. Farghänel and H.-H. Emons, Z. Phys. Chem., 26 (1985) 522.
K. Majima, K. Katsuki et al., Nippon Kaisui Gakkai-Shi., 27 (1974) 321.
W. J. Light Foot and C. F. Pruton, J. Am. Chem. Soc., 69 (1947) 2098.
H. F. Gibbart and A. F. Gossmann Jr., J. Sol. Chem., 3 (1974) 385.
Handbook of Chemistry and Physics, 66th ed. VCRC Press, Bocaraton, Florida 1985-1986.
A. I. Mun and R. S. Darrer, Russ. J. Inorg. Chem., 2 (1957) 658.
W. H. Rodebush, J. Amer. Chem. Soc., 40 (1918) 1204.
H. Stephan, T. Stephan and A. Seidell, Solub. of Inorg. and Org. Comp. 3° ed., New York, 584 (1940) 234.
C. F. Pruton and O. F. Tower, J. Amer. Chem. Soc., 54 (1932) 3040.
B. J. Damilano and S. J. Baabor, Bol. Soc. Chil. Quim., 11 (1961) 3.
N. A. Osokoriva, M. A. Opuikhtina et al., Trans. Inst. State. Applied Chem., USSR, 16 (1932) 24.
H. Dietzel and F. Serow, Frei. Fors., 132(A) (1959) 5.
I. Igelsrud and T. G. Thompson, J. Amer. Chem. Soc., 58 (1936) 318.
W. J. Light Foot and C. F. Pruton, J. Amer. Chem. Soc., 68 (1946) 1.
W. B. Lee and A. C. Egerton, 122 (1923) 706.
K. Majima, K. Katsuki et al., Nippon Kaisui Gakkai-Shi, 26 (1972) 199.
K. Majima, K. Katsuki et al., Nippon Kaisui Gakkai-Shi, 27 (1974) 315.
G. Malquori, Gazz. Chem. Ital., 58 (1928) 891.
E. I. Akhunov and B. B. Wassilyew, Zh. Obshch. Khim., 2 (1932) 271.
K. Majima, K. Katsuki et al., Nippon Kaisui, 27 (1973) 164.
P. Pascal, Nouveau Traité de Chimie Minérale, Masson, Paris 1960.
I. A. Belov, G. I. Novikov and P. K. Rud'ko, Russ. J. Inorg. Chem., 30 (1985) 135.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Atbir, A., Marrouche, A., Atif, H. et al. Calcul Des Diagrammes De Phases Des Systemes Binaires H2O–MCln (M=Mg2+, Fe2+, Fe3+). Journal of Thermal Analysis and Calorimetry 61, 849–860 (2000). https://doi.org/10.1023/A:1010109500891
Issue Date:
DOI: https://doi.org/10.1023/A:1010109500891