Abstract
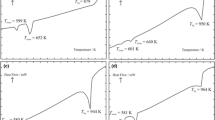
Molar enthalpies of solid-solid and solid-liquid phase transitions of the LaBr3, K2LaBr5, Rb2LaBr5, Rb3LaBr6 and Cs3LaBr6 compounds were determined by differential scanning calorimetry. K2LaBr5 and Rb2LaBr5 exist at ambient temperature and melt congruently at 875 and 864 K, respectively, with corresponding enthalpies of 81.5 and 77.2 kJ mol-1. Rb3LaBr6 and Cs3LaBr6 are the only 3:1 compounds existing in the investigated systems. The first one forms from RbBr and Rb2LaBr5 at 700 K with an enthalpy of 44.0 kJ mol-1 and melts congruently at 940 K with an enthalpy of 46.7 kJ mol-1. The second one exists at room temperature, undergoes a solid-solid phase transition at 725 K with an enthalpy of 9.0 kJ mol-1 and melts congruently at 1013 K with an enthalpy of 57.6 kJ mol-1. Two other compounds existing in the CsBr-based systems (Cs2LaBr5 and CsLa2Br7) decompose peritectically at 765 and 828 K, respectively.
The heat capacities of the above compounds in the solid as well as in the liquid phase were determined by differential scanning calorimetry. A special method - 'step method' developed by SETARAM was applied in these measurements. The heat capacity experimental data were fitted by a polynomial temperature dependence.
Similar content being viewed by others
References
G. N. Papatheodorou and O. J. Kleppa, J. Phys. Chem., 78 (1974) 178.
G. N. Papatheodorou and T. Østwold, J. Phys. Chem., 78 (1974) 181.
M. Gaune-Escard, A. Bogacz, L. Rycerz and W. Szczepaniak, Thermochim. Acta, 236 (1994) 67.
M. Gaune-Escard, A. Bogacz, L. Rycerz and W. Szczepaniak. Thermochim. Acta. 236 (1994) 11.
M. Gaune-Escard, L. Rycerz, W. Szczepaniak and A. Bogacz, J. Alloys Comp., 204 (1994) 189.
M. Gaune-Escard, L. Rycerz, W. Szczepaniak and A. Bogacz, J. Alloys Comp., 204 (1994) 193.
M. Gaune-Escard, A. Bogacz, L. Rycerz and W. Szczepaniak, Thermochim. Acta, 279 (1996) 1.
M. Gaune-Escard, A. Bogacz, L. Rycerz and W. Szczepaniak, Thermochim. Acta, 279 (1996) 11.
SETARAM DSC 121 — Experimentation, B/03NEDSCF.
H. J. Seifert and Y. Yuan, J. Less-Common Metals, 170 (1991) 135.
A. S. Dworkin and M. A. Bredig, High Temp. Sci., 3 (1971) 81.
H. J. Scifert, private information.
O. Knacke, O. Kubaschewski and K. Hesselman, Thermochemical Properties of Inorganic Substances I, Springer, Berlin, 1991, 2nd ed.
I. Barin and O. Knacke, Thermochemical Properties of Inorganic Substances, Springer, Berlin 1973 and 1977.
V. S. Yungman, V. A. Mediviediev, I. V. Veits and G. A. Bergman, IVANTHERMO — A Thermodynamic Database and Software for the Personal Computer, CRC Press and Begel House, Boca Raton, 1993.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rycerz, L., Gaune-Escard, M. Enthalpy of Phase Transitions and Heat Capacity of Stoichiometric Compounds in LaBr3-MBr Systems (M=K, Rb, Cs). Journal of Thermal Analysis and Calorimetry 56, 355–363 (1999). https://doi.org/10.1023/A:1010102802661
Issue Date:
DOI: https://doi.org/10.1023/A:1010102802661