Abstract
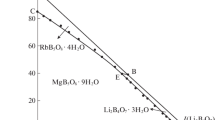
The phase and physicochemical properties diagrams at 323 K of systems Rb+//Cl–, borate–H2O, and Mg2+//Cl–, borate–H2O were plotted using the solubilities, densities, and refractive indices of the systems. The Schreinemakers’ wet residue method and the X-ray diffraction method were used for the determination of the compositions of solid phase. Results show that these two systems belong to the hydrate I type, without solid solution or double salt formation. The phase diagrams of both systems consist of one invariant point, two monovariant curves, and two crystallization zones. The borates formed in the studied systems are RbB5O6(OH)4 · 2H2O, and MgB4O5(OH)4 · 7H2O. The comparison between the stable phase diagrams of the system Rb+//Cl–, borate–H2O at 323 and 348 K shows that no matter at which temperature point, the crystallization form of salts are not changed and the crystallization zones have slightly changed.









Similar content being viewed by others
REFERENCES
F. Q. Cheng, W. T. Cheng, and H. G. Cheng, Basis and Application of Salt Lake Chemical Industry (Science Press, Beijing, 2012).
N. Jaroslav, Solid-Liquid Phase Equilibria (Czechosl. Acad. Sci. Praha, Czech Republic, 1997).
A. V. Churikov, K. V. Zapsis, V. V. Khramkov, M. A. Churikov, and I. A. Kazarinov, J. Chem. Eng. Data 56, 383 (2011).
À. B. Zdanovskii, E. I. Lyakhovskaya, and R. E. Shleimovich, Handbook of Experimental Data on the Solubility of Multicomponent Water–Salt Systems (Goshimizdat, Leningrad, 1953) [in Russian].
H. B. Li, L. Liu, X. D. Yu, Y. J. Zhang, Z. Q. Li, and Y. Zeng, Russ. J. Phys. Chem. A 89, 1572 (2015).
S. Feng, X. D. Yu, X. L. Cheng, and Y. Zeng, Russ. J. Phys. Chem. A 91, 2149 (2017).
X. D. Yu, L. Wang, J. Chen, and M. L. Li, J. Chem. Eng. Data 62, 1427 (2017).
X. D. Yu, Y. L. Luo, L. T. Wu, X. L. Cheng, and Y. Zeng, J. Chem. Eng. Data 61, 3311 (2016).
X. D. Yu, Y. Zeng, S. S. Guo, and Y. J. Zhang, J. Chem. Eng. Data 61, 1246 (2016).
X. D. Yu, Y. Zeng, P. T. Mu, Q. Tan, and D. B. Jiang, Fluid Phase Equilib. 387, 88 (2015).
D. B. Jiang, Y. Zeng, and X. D. Yu, Fluid Phase Equilib. 349, 67 (2013).
X. D. Yu, Y. Zeng, and Z. X. Zhang, J. Chem. Eng. Data 57, 1759 (2012).
X. D. Yu, D. B. Jiang, Q. Tan, and Y. Zeng, Fluid Phase Equilib. 367, 63 (2014).
Q. H. Yin, P. T. Mu, Q. Tan, X. D. Yu, Z. Q. Li, and Y. Zeng, J. Chem. Eng. Data 59, 2235 (2014).
Q. H. Yin, Y. Zeng, X. D. Yu, P. T. Mu, and Q. Tan, J. Chem. Eng. Data 58, 2875 (2013).
Y. T. Lin and Q. Tang, Geol. Chem. Miner. 35, 209 (1999).
Z. Liu, Y. Zeng, and X. D. Yu, Chin. J. Rare Met. 37, 104 (2013).
T. L. Deng, H. Zhou, and X. Chen, Salt–Water System Phase Diagrams and Applications (Chemical Industry Press, Beijing, 2013).
Y. Zeng, X. D. Yu, L. L. Liu, and Q. H. Yin, CN Patent No. 103172078 A (2013).
Y. Jing, Sea-Lake Salt Chem. Ind. 29, 24 (2000).
H. Y. Cheng, and H. Cheng, Chemical Reagent–General Methods for the Determination of Density (China Standards Press, Beijing, 2007).
P. L. Fosbl, K. Thomsen, and E. H. Stenby, J. Solution Chem. 38, 1 (2009).
Inst. of Qinghai Salt-Lake, Chin. Acad. Sci., Analytical Methods of Brines and Salts (Science Technol. Press, Beijing, 1984).
A. F. Wells, Structural Inorganic Chemistry (Oxford Univ. Press, London, 1975).
ACKNOWLEDGMENTS
The present work was supported by the National Natural Science Foundation of China (U1507111, U1607121, 41473059), the Research Fund from the Science and Technology Department of Sichuan Province (2017JY0191), and the Scientific research fund of Sichuan Provincial education department (16ZA0083).
Author information
Authors and Affiliations
Corresponding authors
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Qin Huang, Li, M., Wang, L. et al. The Phase and Physicochemical Properties Diagrams of Systems Rb+(Mg2+)//Cl– and Borate–H2O at 323 K. Russ. J. Phys. Chem. 93, 211–217 (2019). https://doi.org/10.1134/S0036024419020225
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024419020225