Abstract
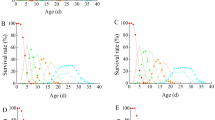
We investigated the use of two insect cell lines to improve an artificial diet (DI) for the pupal ectoparasitoid Diapetimorpha introita. DI was supplemented with Grace's culture medium conditioned with IPL-LdFB, a cell line derived from fat body of the gypsy moth, Lymantria dispar (FBCell diet), and with Grace's medium conditioned with Sf9, a cell line derived from ovaries of the fall armyworm, Spodoptera frugiperda (Sf9Cell diet). The diets were also chemically analyzed for nutrients and any deficiencies were filled by the addition of nutrients. One-half ml aliquots of each diet were encapsulated in paraffin domes and newly hatched larvae of D. introita were placed on each diet (one larva/dome) and allowed to develop to the adult stage. Providing fresh diet on day four when the larvae were in the third instar did not improve parasitoid production. Compared with DI, only Sf9Cell had a positive effect on the parasitoid's growth, increasing the size of male parasitoids. The parasitoids, however, took longer to develop to the adult stage than those reared on the natural host. Neither cell line significantly enhanced the average weight of female parasitoids, shortened developmental time, nor increased % cocoons produced and % adult emergence. Providing additional nutrients (amino acids, vitamins, cations and anions, fatty acids and milk/egg protein) to both diets (based on chemical analyses of the cell line-supplemented diets) enhanced the average weight of the females on Sf9Cell and males and females on FBCell. The nutritional additions, however, did not improve the developmental time, pupation and adult emergence.
Similar content being viewed by others
References
Carpenter, J.E. and P. Greany, 1998. Comparative development and performance of artificially reared vs. host-reared Diapetimorpha introita (Cresson) (Hymenoptera: Ichneumonidae) wasps. Biol. Control 11: 203-208.
Cartwright, T. and G.P. Shah, 1994. Culture media. In: J.M. Davis (ed), Basic Cell Culture — A Practical Approach. IRL Press at Oxford University Press, Oxford. pp. 57-91.
Cho, T., M.L. Shuler and R.R. Granados, 1989. Current developments in new media and cell culture systems for the large-scale production of insect cells. In: Advances In Cell Culture, Vol. 7. Academic Press, San Diego. pp. 261-276.
Ferkovich, S.M., C. Dillard and H. Oberlander, 1991. Stimulation of embryonic development in Microplitis croceipes (Braconidae) in cell culture media preconditioned with a fat body cell line derived from a nonpermissive host, Gypsy moth, Lymantria dispar. Arch. Insect Biochem. Physiol. 18: 169-175.
Ferkovich, S.M. and H. Oberlander, 1991. Stimulation of endoparasitoid egg development by a fat body cell line: activity and characterization of factors that induce germ band formation and hatching. In: Proc. VIII Internat. Conf. Invert. and Fish Tiss. Culture, Tissue Culture Assoc., Columbia, USA. pp. 181-187.
Ferkovich, S.M., H. Oberlander, C. Dillard and E. Leach, 1994. Embryonic development of an endoparasitoid, Microplitis croceipes (Hymenoptera: Braconidae) in cell line-conditioned media. In Vitro Cell Dev. Biol. 30A: 279-282.
Grace, T.D.C., 1962. Establishment of four strains of cells from insect tissue grown in vitro. Nature 195: 788-789.
Greany, P.D., W.R. Clark and S.M. Ferkovich, 1988. Influence of a host hemolymph protein in stimulating early egg development in Microplitis croceipes. Proc. XVIII Int. Congress Entomol., Vancouver, Canada, July 3-9.
Greany, P., W. Clark, S.M. Ferkovich, J. Law and R. Ryan, 1990. Isolation and characterization of a host hemolymph protein required for development of the eggs of the endoparasite Microplitis croceipes. In: H.H. Hagedorn, J.G. Hildebrandi, M.G. Caldwell and J.H. Law (eds), Molecular Insect Science. Plenum Press, New York and London. p. 306.
Greany, P. and J.E. Carpenter, 1996. Culture medium for parasitic and predaceous insects. U.S. Patent 08/692,565: Docket No. 000010.96. (Application pending).
Grenier, S., P.D. Greany and A.C. Cohen, 1994. Potential for mass release of insect parasitoids and predators through development of artificial culture techniques. In: D. Rosen, F.D. Bennett and J.L. Capinera (eds), Pest Management in the Subtropics: Biological Control — a Florida Perspective. Intercept Publishers, Andover, Hampshire, England. pp. 181-205.
Grenier, S., H. Yang, J. Guillaud and L. Chapelle, 1995. Comparative development and biochemical analyses of Trichogramma (Hymenoptera: Trichogrammatidae) grown in artificial media with hemolymph or devoid of insect components. Comp. Biochem. Physiol. 111B: 83-90.
Ha, S.H., T.H. Park and S.E. Kim, 1996. Silkworm hemolymph as a substitute for fetal bovine serum in insect cell culture. Biotech. Techniques 10: 401-406.
Lynn, D.E., E.M. Dougherty, J.T. McClintock and M. Loeb, 1988. Development of cell lines from various tissues of Lepidoptera. In: Y. Kuroda, E. Kurstak and K. Maramorosch (eds), Invertebrate and Fish Tissue Culture. Springer-Verlag, New York. pp. 239-242.
Maiorella, B., D. Inlow, A. Shauger and D. Harano, 1988. Large-scale insect cell-culture for recombinant protein production. Biotechnology 6: 1406-1408.
Mitsuhashi, J. and T. Oshiki, 1993. Preliminary attempts to rear an endoparasitic fly, Exorista sorbillans (Diptera, Tachinidae) in vitro. Japanese Jour. Entom. 61: 459-464.
Nettles, W.C., 1990. In vitro rearing of parasitoids: role of host factors in nutrition. Arch. Insect Bechem. Physiol. 13: 167-175.
Obayashi, T., K. Iwabuchi and J. Mitsuhashi, 1994. In vitro rearing of a larval endoparasitoid, Venturia canescens (Gravenhorst) (Hymenoptera: Ichneumonidae). I. Embryonic development. Appl. Entomol. Zool. 29: 123-126.
Pair, S.D. and H.R. Gross, 1984. Field mortality of pupae of the fall armyworm, Spodoptera frugiperda (J. E. Smith), by predators and a newly discovered parasitoid, Diapetimorpha introita. J. Georgia Entomol. Soc. 19: 22-26.
Pair, S.D. and H.R. Gross, 1989. Seasonal incidence of fall armyworm (Lepidoptera: Noctuidae) pupal parasitism in corn by Diapetimorpha introita and Cryptus albitarsis (Hymenoptera: Ichneumonidae). J. Entomol. Sci. 81: 339-343.
Pair, S.D., 1995. Biology and rearing of Diapetimorpha introita (Cresson) (Hymenoptera: Ichneumonidae) on host and non-host noctuid pupae. J. Entomol Sci. 3: 468-480.
Pennacchio, F.S., B. Vinson and E. Tremblay, 1992. Preliminary results on in vitro rearing of the endoparasitoid Cardiochiles nigriceps from egg to second instar. Entomol. Exper. Appl. 64: 209-216.
Rojas, M.G., J.A. Morales-Ramos, E.G. King, G. Saldana and S.M. Greenberg, 1998. Use of a factitious host and supplemented adult diet to rear and induce oogenesis on Catolaccus grandis (Hymenoptera: Pteromalidae). Environ. Entomol. 27: 499-507.
Rotundo, G., R. Cavalloro and E. Tremblay, 1988. In vitro rearing of Lysiphlebus fabarum (Hym.: Braconidae). Entomophaga 33: 264-267.
Rice, J.W., N.B. Rankle, T.M. Gurganus, C.M. Marr, J.B. Barna, M.M. Walters and D.J. Burns, 1993. A comparison of large-scale Sf 9 insect cell growth and protein production: stirred vessel vs. airlift. Biotechniques 15: 1052-1059.
SAS Institute Inc., 1989. SAS/STAT User's Guide, Version 6, 4th Edn, Vol. 2. SAS Institute Inc., Cary, NC, 846 pp.
Thompson, S.N., 1979. Effect of dietary glucose on in vivo fatty acid metabolism and in vitro synthetase activity in the insect parasite, Exeristes roborator (Fabricius). Insect Biochem. 9: 645-651.
Trager, W., 1935. Cultivation of the virus of grasserie in silkworm tissue cultures. J. Exp. Med. 61: 501-513.
Vinson, S.B. and G.F. Iwantsch, 1980. Host regulation by insect parasitoids. Q. Rev. Biol. 55: 143-165.
Watanabe, M. and J. Mitsuhashi, 1995. In vitro rearing of an endoparasitic fly, Exorista sorbillans (Diptera: Tachinidae). Applied Entomol. and Zool. 30: 319-325.
Wyatt, S.S., 1956. Culture in vitro of tissue from the silk worm, Bombyx mori L. J. Gen. Physiol. 39: 841-852.
Yamamoto, Y., M. Ohori, T. Ohbayashi, K. Iwabuchi and J. Mitsuhashi, 1997. In vitro rearing of the larval endoparasitoid, Venturia canescens (Gravenhorst) (Hymenoptera: Ichneumonidae) II. Larval development. Appl. Entomol. Zool. 32: 256-258.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ferkovich, S., Morales-Ramos, J., Rojas, M. et al. Rearing of ectoparasitoid Diapetimorpha introita on an artificial diet: supplementation with insect cell line-derived factors. BioControl 44, 29–45 (1999). https://doi.org/10.1023/A:1009939028766
Issue Date:
DOI: https://doi.org/10.1023/A:1009939028766