Abstract
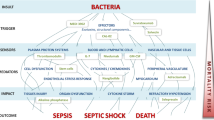
Monoclonal antibodies to tumor necrosis factor alpha (TNF-α) and soluble receptor complexes comprising the extramembrane portion of the TNF receptor coupled with the Fc portion of the human IgG1 molecule have been utilized as therapies for severe sepsis and septic shock. Over 5000 patients have been entered into clinical studies utilizing these two anti-TNF approaches, but no clear benefit in reducing mortality has been shown with such therapies. Although subgroups of infected patients, such as those with elevations in circulating IL-6, appeared to have improved outcome with anti-TNF therapies, no large study has prospectively confirmed such an effect. As a whole, the results for the anti-TNF clinical trials do not provide strong support for this therapeutic approach in broad groups of critically ill patients with sepsis.
Similar content being viewed by others
References
Abraham E, Wunderink R, Silverman H, Perl T, Nasraway S, Levy H, Bone R, Wenzel R, Balk R, Allred R, Pennington J, Wherry J. Monoclonal antibody to human tumor necrosis factor alpha (TNF0 MAb): Efficacy and safety in patients with the sepsis syndrome. JAMA 1995;273:934–941
Ashkenazi A, Marsters SA, Capon DJ, Chamow SM, Figari IS, Pennica D, Goeddel DV, Palladino MA, Smith DH. Protection against endotoxic shock by a tumor necrosis factor receptor immunoadhesin Proc Natl Acad Sci USA 1991; 88:10535–10539
Van Zee KJ, Moldawer LL, Oldenburg HAS, Thompson WZ, Stackpole SA, Montegut WJ, Rogy MA, Meschter C, Gallati H, Schiller CD, et al. Protection against lethal Escherichia coli bacteremia in baboons (Papio anubis) by pretreatment with a 55-kDa TNF receptor (CD120a)-Ig fusion protein, Ro 45-2081 J Immunol 1996;156:2221–2230
Haak-Frendscho M, Marsters SA, Mordenti J, Brady S, Gillett NA, Chen SA, Ashkenazi A. Inhibition of TNF by a TNF receptor immunoadhesin. J Immunol 1994;152: 1347–1353
Sriskandan S, Moyes D, Lemm G, Cohen J. Lymphotoxin-a (TNF-b) during sepsis. Cytokine 1996;8:933–937
Tracey KJ, Fong Y, Hesse DG, Manogue KR, Lee AT, Kuo GC, et al. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteremia. Nature 1987;330: 662–664.
Fong Y, Tracey KJ, Moldawer LL, et al. Antibodies to cachectin/TNF reduce interleukin-1 and interleukin-6 appearance during lethal bacteremia. J Exp Med 1989; 170:1627–1633.
Hinshaw LB, Tekamp-Olson P, Chang AC, et al. Survival of primates in LD100 septic shock following therapy with antibody to tumor necrosis factor (TNF). Circ Shock 1990;30: 279–292.
Hinshaw LB, Emerson TE Jr, Taylor FB Jr, Chang ACK, Duerr M, Peer GT, Flournoy DJ, White GL, Kosanake SD, Murray CK, Xu R, Passey RB, Fournel MA. Lethal S. aureus shock in primates: prevention of death with anti-TNF antibody. J Trauma 1992;33:568–573.
Fisher CJ, Opal SM, Dhainaut J-F, Stephens S, Zimmerman JL, Nightingale P, Harris SJ, Schein RMH, Panacek EA, Vincent J-L, et al. Influence of an anti-tumor necrosis factor monoclonal antibody on cytokine levels in patients with sepsis Crit Care Med 1993;21:318–327
Dhainaut J-F A, Vincent J-L, Richard C, Lejeune P, Martin C, Fierobe L, Stephens S, Ney UM, Sopwith M. CDP571, a humanized antibody to human tumor necrosis factor-a: Safety, pharmacokinetics, immune response, and influence of the antibody on cytokine concentrations in patients with septic shock Crit Care Med 1995;23:1461–1469
Cohen J, Carlet J, for the INTERSEPT Study Group. INTERSEPT: An international, multicenter, placebo-controlled trial of monoclonal antibody to human tumor necrosis factor-a in patients with sepsis. Crit Care Med 1996;24: 1431–1440
Abraham E, Anzueto A, Gutierrez G, et al. Double-blind randomized controlled trial of monoclonal antibody to human tumor necrosis factor in treatment of septic shock. Lancet 1998;351:929–933
Reinhart K, Wiegand-Lohnert C, Grimminger F, Kaul M, Withington S, Treacher D, Eckart J, Willatts S, Bouza C, Krausch D, et al. Assessment of the safety and efficacy of the monoclonal anti-tumor necrosis factor antibody-fragment, MAK 195F, in patients with sepsis and septic shock: A multicenter, randomized, placebo-controlled, dose-ranging study. Crit Care Med 1996;24:733–742
Fisher CJ, Agosti JM, Opal SM, Lowry SF, Balk RA, Sadoff JC, Abraham E, Schein RMH, Benjamin E, and the Soluble TNF Receptor Sepsis Study Group. Treatment of patients with septic shock with tumor necrosis factor receptor Fc fusion protein. N Engl J Med 1996;334:1697–1702.
Abraham E, Glauser MP, Butler T, Lew D, Gelmont D, Laterre PF, Kudsk K, Bruining HA, Otto C, Tobin E, Zwingelstein C, Lesslauer W, Leighton A. p55 tumor necrosis factor receptor fusion protein in the treatment of patients with severe sepsis and septic shock: A placebo controlled, randomized, double-blind, multi-center clinical trial. JAMA 1997;277:1531–1538
Abraham E. Presented at the American Thoracic Society International Conference, April 28, 1998.
Evans TJ, Moyes D, Carpenter A, Martin R, Loetscher H, Lesslauer W, Cohen J. Protective effect of 55-but not 75-kD soluble tumor necrosis factor receptor-immunoglobulin G fusion proteins in an animal model of gram-negative sepsis. J Exp Med 1994;180:2173–2179
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Abraham, E. Anti-TNF Therapies. Sepsis 3, 47–50 (1999). https://doi.org/10.1023/A:1009875126060
Issue Date:
DOI: https://doi.org/10.1023/A:1009875126060