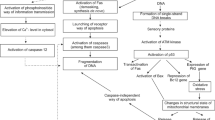
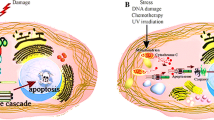
Abstract
We investigated changes typical for apoptosis in various cell lines after UV-B irradiation. Using established methods for detection of apoptosis we demonstrate changes of cellular morphology, phosphatidylserine (PS) exposure, ollgonucleosomal DNA fragmentation and generation of hypochrome nuclei. To isolated high-molecular-weight (hmwt) DNA fragments we engaged a new method avoiding pulse field gel electrophoresis. Most UV-B irradiated cell lines showed oligonucleosomal DNA fragmentation, hypochrome nuclei, morphological changes, annexin-V binding and positive TUNEL reaction. However, no oligonucleosomal DNA fragmentation could be detected in Raji and HaCaT cells. Whereas HaCaT cells displayed all other changes typical for apoptosis, Raji cells were TUNEL negative, formed low amounts of hmwt DNA and showed an 'atypically' low hypochrome shift. Nevertheless, UV-B irradiated Raji cells excluded propidium iodide (PI), bound annexin-V and stopped proliferation. This suggests that Raji cells underwent growth arrest with exposure of PS being the only feature of apoptosis. However, in the presence of phagocytes expressing the phosphatidylserine receptor these cells would share the removal pathway with apoptotic cells. Since UV-B induced programmed cell death differs in dependence of cells under investigation, the failure to detect oligonucleosomal DNA fragmentation or chromatin condensation is not suitable to exclude programmed (apoptotic?) cell death.
Similar content being viewed by others
References
Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol 1980; 68: 251–306.
Allan DJ, Gobe GC, Harmon BV. Sertoli cell death by apoptosis in the immature rat testis following x-irradiation. Scanning Microsc 1988; 2: 503–512.
Hendry JH, Potten CS. Intestinal cell radiosensitivity: a comparison for cell death assayed by apoptosis or by a loss of clonogenicity. Int J Radiat Biol Relat Stud Phys Chem Med 1982; 42: 621–628.
Ritke MK, Rusnak JM, Lazo JS, et al. Differential induction of etoposide-mediated apoptosis in human leukemia HL-60 and K562 cells. Mol Pharmacol 1994; 46: 605–611.
Hartmann A, Blaszyk H, Cunningham JS, et al. Overexpression and mutations of p53 in metastatic malignant melanomas. Int J Cancer 1996; 67: 313–317.
Manome Y, Yao XJ, Kufe DW, Cohen EA, Fine HA. Selective effects of DNA damaging agents on HIV long terminal repeat activation and virus replication in vitro. J Acquir Immune Defic Syndr Hum Retrovirol 1996; 11: 109–116.
Norval M, el Ghorr A, Garssen J, Van Loveren H. The effects of ultraviolet light irradiation on viral infections. Br J Dermatol 1994; 130: 693–700.
Panozzo J, Panozzo J, Akan E, Libertin C, Woloschak GE. The effects ofcisplatin and methotrexate on the expression of human immunodeficiency virus type 1 long terminal repeat. Leuk Res 1996; 20: 309–317.
Yarosh DB, Kripke ML. DNA repair and cytokines in antimutagenesis and anticarcinogenesis. Mutat Res 1996; 350: 255–260.
Tobin D, Nilsson M, Toftgard R. Ras-independent activation of Rel-family transcription factors by UVB and TPA in cultured keratinocytes. Oncogene 1996; 12: 785–793.
Yang YM, Dolan LR, Ronai Z. Expression of dominant negative CREB reduces resistance to radiation of human melanoma cells. Oncogene 1996; 12: 2223–2233.
Brown SB, Kluck RM, Ellem KA. Loss and shedding of surface markers from the leukemic myeloid monocytic line THP-I induced to undergo apoptosis. J Cell Biochem 1996; 60: 246–259.
Li G, Mitchell DL, Ho VC, Reed JC, Tron VA. Decreased DNA repair but normal apoptosis in ultraviolet-irradiated skin of p53-transgenic mice. Am J Pathol 1996; 148: 1113–1123.
Schwarz A, Bhardwaj R, Aragane Y, et al. Ultraviolet-B-induced apoptosis of keratinocytes: evidence for partial involvement of tumor necrosis factor-alpha in the formation of sunburn cells. J Invest Dermatol 1995; 104: 922–927.
Yaron I, Yaron R, Oluwole SF, Hardy MA. UVB irradiation of human-derived peripheral blood lymphocytes induces apoptosis but not T-cell anergy: additive effects with various immunosuppressive agents. Cell Immunol 1996; 168: 258–266.
Herrmann M, Lorenz HM, Voll R, Grunke M, Woith W, Kalden JR. A rapid and simple method for the isolation of apoptotic DNA fragments. Nucleic Acids Res 1994; 22: 5506–5507.
Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods 1991; 139: 271–279.
Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol 1988; 106: 761–771.
Bromidge TJ, Howe DJ, Johnson SA, Phillips MJ. Adaptation of the TdT assay for semi-quantitative flow cytometric detection of DNA strand breaks. Cytometry 1995; 20: 257–260.
Vermes I, Haanen C, Steffens Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods 1995; 184: 39–51.
Elstein KH, Zucker RM. Comparison of cellular and nuclear flow cytometric techniques for discriminating apoptotic subpopulations. Exp Cell Res 1994; 211: 322–331.
Brancolini C, Benedetti M, Schneider C. Microfilament reorganization during apoptosis: the role of Gas2, a possible substrate for ICE-like proteases. EMBO J 1995; 14: 5179–5190.
Martin SJ, Cotter TG. Disruption of microtubules induces an endogenous suicide pathway in human leukaemia HL-60 cells. CeLl Tissue Kinet 1990; 23: 545–559.
Martin SJ, Reutelingsperger CP, McGahon AJ, et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med 1995; 182: 1545–1556.
Rusnak JM, Calmels TP, Hoyt DG, Kondo Y, Yalowich JC, Lazo JS. Genesis of discrete higher order DNA fragments in apoptotic human prostatic carcinoma cells. Mol Pharmacol 1996; 49: 244–252.
Wu L, Lanfear J, Harrison PR. The selenium metabolite selenodiglutathione induces cell death by a mechanism distinct from H2O2 coxicity. Carcinogenesis 1995; 16: 1579–1584.
Weis M, Schlegel J, Kass GE, et al. Cellular events in Fas/APO-1-mediated apoptosis in JURKAT T lymphocytes. Exp Cell Res 1995; 219: 699–708.
Walker PR, Weaver VM, Lach B, LeBlanc J, Sikorska M. Endonuclease activities associated with high molecular weight and internucleosomal DNA fragmentation in apoptosis. Exp Cell Res 1994; 213: 100–106.
Weaver VM, Lach B, Walker PR, Sikorska M. Role of proteolysis in apopcosis: involvement of serine proteases in intemucleosomal DNA fragmentation in immature thymocytes. Biochem Cell Biol 1993; 71: 488–500.
Oberhammer F, Wilson JW, Dive C, et al. Apoptotic death in epithelial cells: cleavage of DNA 300 and/or 50 kb fragments prior to or in the absence of internucleosomal fragmentation. EMBO J 1993; 12: 3679–3684.
Walker PR, Kokileva L, LeBlanc J, Sikorska M. Detection of the initial stages of DNA fragmentation in apoptosis. Biotechniques 1993; 15: 1032–1040.
Watanabe H, Kanbe K, Shinozaki T, Hoshino H, Chigira M. Apoptosis of a fibrosarcoma induced by protein-free culture involves DNA cleavage to large fragments but not inrernucleosomal fragmentation. Int J Cancer 1993; 62: 191–198.
Wong P, Smith SB, Bora N, Gentleman S. The use of C0t-1 probe DNA for the detection of low levels of DNA fragmentation. Biochem Cell Biol 1994; 72: 649–653.
Hickish T, Robertson D, Clarke P, et al. Ultrastructural localization of BHRF1: an Epstein-Barr virus gene product which has homology with bcl-2. Cancer Res 1994; 54: 2808–2811.
Dole MG, Jasty R, Cooper MJ, Thompson CB, Nunez G, Cascle VP. Bcl-xL is expressed in neuroblastoma cells and modulates chemotherapy-induced apoptosis. Cancer Res 1995; 55: 2576–2582.
Telford WG, King LE, Fraker PJ. Rapid quantitation of apoptosis in pure and heterogeneous cell populations using flow cytomecry. J Imunnol Methods 1994; 172: 1–16.
Lizard G, Fournel S, Genestier L, et al. Kinetics of plasma membrane and mitochondrial alterations in cells undergoing apopcosis. Cytometry 1995; 21: 275–283.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hagenhofer, M., Germaier, H., Hohenadl, C. et al. UV-B Irradiated Cell Lines Execute Programmed Cell Death in Various Forms. Apoptosis 3, 123–132 (1998). https://doi.org/10.1023/A:1009601109509
Issue Date:
DOI: https://doi.org/10.1023/A:1009601109509