Abstract
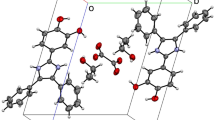
A di(μ-oxo)-bridged dinuclear complex, [VO2(pamh)]2 was isolated by reacting bis(acetylacetonato)vanadium(IV) and the Schiff base, N-(anisoyl)-N′-(picolinylidene)-hydrazine (Hpamh) in acetonitrile. The complex crystallizes in the space group \(P\bar 1 \) ;1; (#2) on crystallographic inversion center. Crystal data: a = 8.2202(12) Å, b = 9.8389(19) Å, c = 10.1907(17) Å, α = 68.245(15)°, β = 74.47(2)°, γ = 66.710(19)°, V = 696.0(2) Å3, and Z = 1. The physical properties of the complex and the structural parameters are consistent with the +5 oxidation state of the metal ions. The monomeric VO2(pamh) unit is square-pyramidal. The planar mononegative ligand (pamh−) coordinates the metal ion via the pyridine-N, the imine-N, and the amide-O atoms. One of the oxo groups completes the NNOO basal plane and also participates in the Vndash;Ondash ;V bridge formation. The other oxo group satisfies the fifth apical coordination site. The molecular structure of the dimeric complex, [VO2(pamh)]2 can be described as two edge-shared distorted VO4N2 octahedra.
Similar content being viewed by others
References
Casellato, U.; Vigato, P.A.; Vidali, M. Coord. Chem. Rev. 1977, 23, 31.
Syamal A.; Maurya, M.R. Coord. Chem. Rev. 1989, 95, 183.
Sangeetha, N.R.; Baradi, K.; Gupta, R.; Pal, C.K.; Manivannan, V.; Pal, S. Polyhedron 1999, 18, 1425.
Sangeetha, N.R.; Pal, S. J. Chem. Crystallogr. 1999, 29, 287.
Rowe, R.A.; Jones, M.M. Inorg. Synth. 1957, 5, 113.
Xtal3.4 User's Manual; Hall, S.R.; King, G.S.D.; Stewart, J.M., Eds.; University of Western Australia: Lamb, Perth, 1995.
Sheldrick, G.M. SHELX-97, Structure Determination Software; University of Göttingen: Göttingen, Germany, 1997.
McArdle, P. J. Appl. Crystallogr. 1995, 28, 65.
Spek, A.L. Platon98, Molecular Graphics Software; University of Glasgow: UK, 1998.
Kemp, W. Organic Spectroscopy; ELBS/Macmillan: Hong Kong, 1987; p 65.
Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; Wiley: New York, 1986; p 244.
El-Sayed, L.; Iskander, M.F. J. Inorg. Nucl. Chem. 1971, 33, 435.
Choudhury, S.; Kakoti, M.; Deb, A.K.; Goswami, S. Polyhedron 1992, 11, 3183.
Li, X.; Lah, M.S.; Pecoraro, V.L. Inorg. Chem. 1988, 27, 4657.
Zhang, X.-M.; You, X.-Z. Polyhedron 1996, 15, 1793.
Choudhury, A.; Geetha, B.; Sangeetha, N.R.; Kavita, V.; Susila, V.; Pal, S. J. Coord. Chem. 1999, 48, 87.
Kojima, A.; Okazaki, K.; Ooi, S.; Saito, K. Inorg. Chem. 1983, 22, 1168.
Rath, S.P.; Mondal, S.; Chakravorty, A. Inorg. Chim. Acta 1997, 263, 247.
Sangeetha, N.R.; Pal, S. Bull. Chem. Soc. Jpn. 2000, 73, 357.
Meichang, S.; Xun, D.; Yougi, Y. Sci. Sin. Ser. B (Engl. Ed.) 1988, 31, 789.
Mokry, L.M.; Carrano, C.J. Inorg. Chem. 1993, 32, 6119.
Duncan, C.A., Copeland, E.P., Kahwa, I.A., Quick, A.; Williams, D.J. J. Chem. Soc., Dalton Trans. 1997, 917.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pal, S., Pal, S. A dimeric pervanadyl (VO2+) complex with a tridentate Schiff base ligand. Journal of Chemical Crystallography 30, 329–333 (2000). https://doi.org/10.1023/A:1009561224540
Issue Date:
DOI: https://doi.org/10.1023/A:1009561224540