Abstract
Bis-[2-(3,4-dihydroxyphenyl)-4,5-diphenyl-1H-imidazol-3-ium] Oxalate ethanol solvate crystal has been isolated from slow solvent evaporation method and the structure was characterized by FT-IR, NMR and Single crystal XRD. The compound crystallizes in the triclinic space group P \(\overline{1 }\) with a = 7.7925(16), b = 10.716(3), c = 13.952(3), α = 106.545(5), β = 97.514(5), γ = 110.152(5), V = 1014.0(4) Å3 and Z = 1. The single crystal X-ray data of the compound confirms two proton transfers from an oxalic acid to the pyrimidine-type-nitrogen of two separate imidazole rings. The structure and symmetry of the Imidazolium Oxalate is dictated by N–H⋯O and O–H⋯O hydrogen bonding interactions and are confirmed by hydrogen bonding analysis and hirshfeld surface analysis. The partial double bond character in the imidazolium ring confirms delocalization across the molecular framework. The partial double bond character of the C–O bonds also confirms delocalization in the oxalate anion. The crystal is 3-dimensional structure, with crystal growth in all the crystallographic axis. Computational analysis [DFT, B3LYP/6-311G(d,p)] reveals close correlation of the constrained optimized structure with the experimental data.
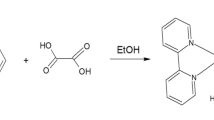
Graphical Abstract
Imidazolium oxalate crystal form by the protonation of Imidazole compound and complex formation with oxalate ion.









Similar content being viewed by others
Data Availability
The authors confirm that the data supporting the findings of this study are available in the supplementary materials and crystal data is available in CCDC. (https://www.ccdc.cam.ac.uk/structures/Search?access=referee&ccdc=2245593&Author=Peter+solo).
References
Bosshard C, Spreiter R, Meier U et al (1999) Organic Materials for Second-Order Nonlinear Optic. In: Dario B, Fabrizia G (eds) Crystal Engineering From Molecules and Crystals to Materials. Springer, Dordrecht
Farchioni R, Grosso G (2001) Organic electronic materials. Springer, Berlin
Kwak HS, An Y, Giesen DJ et al (2022) Design of organic electronic materials with a goal-directed generative model powered by deep neural networks and high-throughput molecular simulations. Front Chem. https://doi.org/10.3389/fchem.2021.800370
Kuo C-J, Li T-Y, Lien C-C et al (2009) Bis(phenanthroimidazolyl)biphenyl derivatives as saturated blue emitters for electroluminescent devices. J Mater Chem 19:1865. https://doi.org/10.1039/b816327h
Wang ZM, Song XH, Gao Z et al (2012) Tuning of the electronic and optical properties of 4,4’-bis(1-phenyl-phenanthro[9,10-d]imidazol-2-yl)biphenyl via cyano substitution in un-conjugated phenyl. RSC Adv 2:9635. https://doi.org/10.1039/c2ra21054a
Wang Z, Li X, Xue K et al (2016) Towards stable deep-blue emission and low efficiency roll-off in OLEDs based on phenanthroimidazole dimers. J Mater Chem C Mater 4:1886–1894. https://doi.org/10.1039/C5TC04048E
Zhang Y, Lai S-L, Tong Q-X et al (2012) High efficiency nondoped deep-blue organic light emitting devices based on imidazole-π-triphenylamine derivatives. Chem Mater 24:61–70. https://doi.org/10.1021/cm201789u
Zhang H, Li A, Li G et al (2020) Achievement of high-performance nondoped blue OLEDs based on AIEgens via construction of effective high-lying charge-transfer state. Adv Opt Mater 8:1902195. https://doi.org/10.1002/adom.201902195
Zhang Y, Lai S-L, Tong Q-X et al (2011) Synthesis and characterization of phenanthroimidazole derivatives for applications in organic electroluminescent devices. J Mater Chem 21:8206. https://doi.org/10.1039/c1jm10326a
Huang Z, Xiang S, Zhang Q et al (2018) Highly efficient green organic light emitting diodes with phenanthroimidazole-based thermally activated delayed fluorescence emitters. J Mater Chem C Mater 6:2379–2386. https://doi.org/10.1039/C7TC05576E
Xu Y, Liang X, Liang Y et al (2019) Efficient deep-blue fluorescent OLEDs with a high exciton utilization efficiency from a fully twisted phenanthroimidazole-anthracene emitter. ACS Appl Mater Interfaces 11:31139–31146. https://doi.org/10.1021/acsami.9b10823
Huang H, Wang Y, Zhuang S et al (2012) Simple phenanthroimidazole/carbazole hybrid bipolar host materials for highly efficient green and yellow phosphorescent organic light-emitting diodes. J Phys Chem C 116:19458–19466. https://doi.org/10.1021/jp305764b
Liao Y-L, Lin C-Y, Wong K-T et al (2007) A novel ambipolar spirobifluorene derivative that behaves as an efficient blue-light emitter in organic light-emitting diodes. Org Lett 9:4511–4514. https://doi.org/10.1021/ol701994k
Chen C-H, Huang W-S, Lai M-Y et al (2009) Versatile, benzimidazole/amine-based ambipolar compounds for electroluminescent applications: single-layer, blue, fluorescent OLEDs, hosts for single-layer, phosphorescent OLEDs. Adv Funct Mater 19:2661–2670. https://doi.org/10.1002/adfm.200900561
Lai M-Y, Chen C-H, Huang W-S et al (2008) Benzimidazole/amine-based compounds capable of ambipolar transport for application in single-layer blue-emitting OLEDs and as hosts for phosphorescent emitters. Angew Chem Int Ed 47:581–585. https://doi.org/10.1002/anie.200704113
Yang X, Zheng S, Bottger R et al (2011) Efficient fluorescent deep-blue and hybrid white emitting devices based on carbazole/benzimidazole compound. J Phys Chem C 115:14347–14352. https://doi.org/10.1021/jp203115c
Zhang Z, Zhang H, Jiao C et al (2015) 2-(2-Hydroxyphenyl)benzimidazole-based four-coordinate boron-containing materials with highly efficient deep-blue photoluminescence and electroluminescence. Inorg Chem 54:2652–2659. https://doi.org/10.1021/ic502815q
Zhang T, Zhang R, Zhao Y, Ni Z (2018) A new series of N -substituted tetraphenylethene-based benzimidazoles: aggregation-induced emission, fast-reversible mechanochromism and blue electroluminescence. Dyes Pigm 148:276–285. https://doi.org/10.1016/j.dyepig.2017.09.018
Lee J, Shizu K, Tanaka H et al (2015) Controlled emission colors and singlet–triplet energy gaps of dihydrophenazine-based thermally activated delayed fluorescence emitters. J Mater Chem C Mater 3:2175–2181. https://doi.org/10.1039/C4TC02530J
Takizawa S, Montes VA, Anzenbacher P (2009) Phenylbenzimidazole-based new bipolar host materials for efficient phosphorescent organic light-emitting diodes. Chem Mater 21:2452–2458. https://doi.org/10.1021/cm9004954
Gong S, Zhao Y, Yang C et al (2010) Tuning the photophysical properties and energy levels by linking spacer and topology between the benzimidazole and carbazole units: bipolar host for highly efficient phosphorescent OLEDs. J Phys Chem C 114:5193–5198. https://doi.org/10.1021/jp100034r
Ge Z, Hayakawa T, Ando S et al (2008) Spin-coated highly efficient phosphorescent organic light-emitting diodes based on bipolar triphenylamine-benzimidazole derivatives. Adv Funct Mater 18:584–590. https://doi.org/10.1002/adfm.200700913
Priya BS, RajanBabu D (2023) Structural, optical and mechanical properties of Di Imidazolium oxalate monohydrate single crystals. J Mol Struct 1291:135980. https://doi.org/10.1016/j.molstruc.2023.135980
Callear SK, Hursthouse MB, Threlfall TL (2010) A systematic study of the crystallisation products of a series of dicarboxylic acids with imidazole derivatives. CrystEngComm 12:898–908. https://doi.org/10.1039/B917191F
Rachocki A, Pogorzelec-Glaser K, Tritt-Goc J (2008) 1H NMR relaxation studies of proton-conducting imidazolium salts of dicarboxylic acids. Appl Magn Reson 34:163–173. https://doi.org/10.1007/s00723-008-0088-6
Dolomanov OV, Bourhis LJ, Gildea RJ et al (2009) OLEX2: a complete structure solution, refinement and analysis program. J Appl Crystallogr 42:339–341. https://doi.org/10.1107/S0021889808042726
Sheldrick GM (2008) A short history of SHELX. Acta Crystallogr A 64:112–122. https://doi.org/10.1107/S0108767307043930
Sheldrick GM (2015) Crystal structure refinement with SHELXL. Acta Crystallogr C Struct Chem 71:3–8. https://doi.org/10.1107/S2053229614024218
Frisch CJ, Trucks GW, Schlegel HB et al (2009) Gaussian 09, Revision A.02
Spackman PR, Turner MJ, McKinnon JJ et al (2021) CrystalExplorer: a program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J Appl Crystallogr 54:1006–1011. https://doi.org/10.1107/S1600576721002910
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592. https://doi.org/10.1002/jcc.22885
Jeffrey GA, Parry GS (1952) 947 The structure of the oxalate ion. J Chem Soc. https://doi.org/10.1039/jr9520004864
Xi N, Huang Q, Liu L (2008) Comprehensive heterocyclic chemistry. Elsevier, Hoboken
Acknowledgements
The authors are grateful to St. Joseph’s College (A) Jakhama, for providing all the facilities to perform the research work. The authors also acknowledge SAIC, Tezpur University, for the high-quality single crystal XRD data collection.
Author information
Authors and Affiliations
Contributions
Peter Solo: conceptualization, original draft, writing, software, validation, investigation. M. Arockia doss: methodology, supervision, review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Solo, P., Arockia doss, M. Synthesis, Crystal Structure, Hirshfeld Surface and Computational Analysis of Bis-[2-(3,4-dihydroxyphenyl)-4,5-diphenyl-1H-imidazol-3-ium] Oxalate Ethanol Solvate. J Chem Crystallogr (2024). https://doi.org/10.1007/s10870-024-01016-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10870-024-01016-3