Abstract
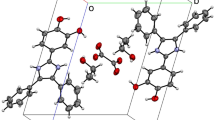
Crystal structure of inclusion complex of 5, 17-di-t-butyl-26, 28-disubstituted calix[4]arene 3 with acetone, [(C54H58N2O6·CH3COCH3)·2CH3COCH3], was determined by X-ray crystallographic analysis. It possesses space group C2/c, with a = 26.277(8), b = 12.967(5), c = 20.355(4) Å, β = 124.845(14)°, and D calc = 1.173 mg/m3 for Z = 4. Crystal data indicate that action of one molecule of compound 3 upon three molecules of acetone forms a clathrate intermolecular inclusion complex.
Similar content being viewed by others
References
Vicens, J.; Bhömer, V. Calixarenes—AVersatile Class of Macrocyclic Compounds; Dordrecht: Kluwer, 1991.
Pochini, A.; Ungaro, R. In Comprehensive Supramolecular Chemistry: Calix[4]arenes and Related Hosts; Lehn, J.M.; Vögtle, F., Eds.; Pergamon: New York, 1996; pp 103–142.
Andreetti, G.D.; Ungaro, R.; Pochini, A. J. Chem. Soc. Chem. Commun. 1979, 1005.
Perrin, M.; Gharnati, F.; Oehler, D.; Perrin, R.; Lecocq, S. J. Inclusion Phenom. Mol. Recognit. 1992, 14, 257.
Ungaro, R.; Pochini, A. J. Chem. Soc. Perkin Trans. II 1984, 1979.
Zhang, P-M.; Huang, Z-T. Acta Chim. Sin. 1992, 50, 209.
Brouwer, E.B.; Udachin, K.A.; Enright, G.D.; Ratcliffe, C.I.; Ripmeester, J.A. Chem. Commun. 1998, 5, 587.
Thuery, P.; Keller, N.; Lance, M.; Vigner, J-D.; Nierlich, M. J. Inclusion Phenom. Mol. Recognit. Chem. 1995, 20, 373.
Arduin, A.; McGregor, W.M.; Paganuzzi, D.; Pochini, A.; Secchi, A.; Ugozzoli, F.; Ungaro, R. J. Chem. Soc. Perkin Trans. II 1996, 839.
Brouwer, E.B.; Enright, G.D.; Ripmeester, J.A. Supramol. Chem. 1996, 7, 7.
Bohmer, V.; Ferguson, G.; Frings, M. Acta Crystallogr. Sect. C: Cryst. Struct. Commun. 1997, 53, 1293.
Van Loon, J-D.; Arduin, A.; Coppi, L.; Verboom, W.; Pochini, A.; Ungaro, R.; Harkema, S; Reinhoudt, D.N. J. Org. Chem. 1990, 55, 5639.
XSCANS (version 2.1), Siemens Analytical X-ray Instruments: Madison, WI, 1994.
SHELXTL (version 5.0), Reference Manual, Siemens Industrial Automation, Analytical Instrumentation: Madison, WI, 1995.
Andreetti, G.D.; Ori, F.O.; Ugozzoli, C.; Alfieri, A.; Pochini, A.; Ungaro, R. J. Inclusion Phenom. Mol. Recognit. Chem. 1998, 6, 523.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lu, G., Liu, F., Liu, Y. et al. Crystal structure of the molecular inclusion complex of 5, 17-di-t-butyl-26, 28-disubstituted calix[4]arene with acetone (1:3). Journal of Chemical Crystallography 29, 1121–1125 (1999). https://doi.org/10.1023/A:1009514026129
Issue Date:
DOI: https://doi.org/10.1023/A:1009514026129