Abstract
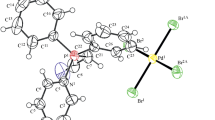
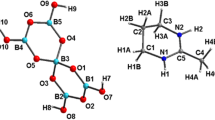
Ten-vertex [6,6-(PMe2Ph)2-arachno-6-PdB9H12-9-(PMe2Ph)] 1a and eleven-vertex [7,7-(PMe2Ph)2-nido-7-PdB10H12] 2a have been isolated as occasional by-products from an extension to palladaborane chemistry of the one-pot route for the preparation of the nine-vertex platinaborane [(PMe2Ph)2PtB8H12] 3b from [PtCl2(PMe2Ph)2] and B10H14, but using [PdCl2(PMe2Ph)2] instead of [PtCl2(PMe2Ph)2] to generate [4,4-(PMe2Ph)2-arachno-4-PdB8H12] 3a in yields of upto 75%. The two by-products 1a and 2a are each characterized by single-crystal X-ray diffraction analysis. Space group and cell parameters are as follows: for 1a, triclinic, \(P\bar 1 \) , a = 10.1139(2) Å, b = 10.3955(2) Å, c = 31.5086(7) Å, α = 94.4720(12)°, β = 91.6420(10)°, and γ = 105.3790(11)°; for 2a, monoclinic, P21/n, a = 14.6059(3) Å, b = 10.9988(2) Å, c = 15.1516(3) Å, and β = 93.0000(14)°. Whereas 1a and 1b are isomorphous, Compounds 2a and 2b are not, and show significant differences in intramolecular conformation.
Similar content being viewed by others
References
Kennedy, J.D. Prog. Inorg. Chem. 1984, 32, 519.
Kennedy, J.D. Prog. Inorg. Chem. 1986, 34, 211.
Barton, L.; Srivastava, D. In Comprehensive Organometallic Chemistry II; Wilkinson, G.; Abel, E.W.; Stone, F.G.A., Eds.; Pergamon: New York and London 1995; Vol. 1, Chapter 8, pp 275-372.
Bould, J.; Clegg, W.; Teat, S.J.; Barton, L.; Rath, N.P.; Thornton-Pett, M.; Kennedy, J.D. Inorg. Chim. Acta 1999, 289, 95.
Boocock, S.K.; Greenwood, N.N.; Kennedy, J.D.; McDonald, W.S.; Staves, J. J. Chem. Soc.; Dalton Trans. 1981, 2573.
Kim, Y.-H.; Brownless, A.; Cooke, P.A.; Greatrex, R.; Kennedy, J.D.; Thornton-Pett, M. Inorg. Chem. Commun. 1998, 1, 19.
Williams, R.E. Inorg. Chem. 1971, 10, 210; Adv. Inorg. Chem. Radiochem. 1976, 18, 67.
Wade, K. Chem. Commun. 1971, 792; Adv. Inorg. Chem. Radiochem. 1976, 18, 1.
Greenwood, N.N.; Hails, M.J.; Kennedy, J.D.; McDonald, W.S. J. Chem. Soc.; Dalton Trans. 1985, 953.
Boocock, S.K.; Greenwood, N.N.; Hails, M.J.; Kennedy, J.D.; McDonald, W.S. J. Chem. Soc.; Dalton Trans. 1981, 1415.
Kennedy, J.D.; Wrackmeyer, B. J. Magn. Reson. 1980, 38, 529.
Guggenberger, L.J.; Kane, A.R.; Muetterties, E.L. J. Am. Chem. Soc. 1972, 94, 5665.
Beckett, M.A.; Crooke, J.E.; Greenwood, N.N.; Kennedy, J.D. J. Chem. Soc.; Dalton Trans. 1984, 1427.
Bould, J.; Crooke, J.E.; Greenwood, N.N.; Kennedy, J.D. J. Chem. Soc.; Dalton Trans. 1984, 190.
Faridoon; Ni Dhubhghaill, O.; Spalding, T.R; Ferguson, G.; Fontaine, X.L.R.; Kennedy, J.D. J. Chem. Soc.; Dalton Trans. 1989, 1657.
Bould, J.; Kennedy, J.D.; McDonald, W.S. Inorg. Chim. Acta 1992, 196, 201.
Bould, J.; Cooke, P.A.; Dörfler, U.; Kennedy, J.D.; Barton, L.; Rath, N.P.; Thornton-Pett, M. Inorg. Chim. Acta 1999, 285, 290.
Boocock, S.K.; Greenwood, N.N.; Kennedy, J.D. J. Chem. Soc.; Chem. Commun. 1980, 305.
Blessing, R.H. Acta Crystallogr. 1995, A51, 33.
COLLECT; Data Collection Strategy Program, Nonius BV, Delft, The Netherlands, 1999.
Otwinowski, Z.; Minor, W. Methods Enzymol. 1997, 276, 307-326.
Sheldrick, G.M. Acta Crystallogr. 1990, A46, 467.
Sheldrick, G.M. SHELXL 97, Program System for Refining Crystal Structures; University of Göttingen: Germany, 1997.
McArdle, P. J. Appl. Cryst. 1995, 28, 65.
Thornton-Pett, M. WINCIF, A WindowsTM CIF Editor; University of Leeds: UK, 2000.
Reed, D. Chem. Soc. Rev. 1993, 22, 109, and references therein.
Ferguson, G.; Kennedy, J.D.; Fontaine, X.L.R.; Faridoon; Spalding, T.R. J. Chem. Soc.; Dalton Trans. 1988, 2555.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Londesborough, M.G., O'Dowd, C., Bould, J. et al. Polyhedral palladaborane chemistry: isolation and structural characterization of ten-vertex [(PMe2Ph)2PdB9H12(PMe2Ph)] and eleven-vertex [(PMe2Ph)2PdB10H12]. Journal of Chemical Crystallography 30, 283–289 (2000). https://doi.org/10.1023/A:1009503405885
Issue Date:
DOI: https://doi.org/10.1023/A:1009503405885