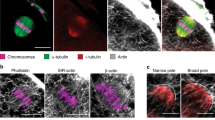
Abstract
Living crane-fly spermatocytes were treated with 10–20 μg/ml cytochalasin D (CD) or 0.3 μg/ml latrunculin (LAT) at various stages of meiosis I. The drugs had the same effects on chromosome behaviour, but CD effects were reversible and LAT effects generally were not. When applied in mid-prometaphase to metaphase, both drugs altered subsequent anaphase poleward movements: half-bivalents either moved more slowly than normal, or moved more slowly after a brief period of movement at normal rate or stalled for 10 min or more immediately after disjunction. CD effects were reversible: within 1 min after washing out the CD, stopped chromosomes started moving and slowed chromosomes sped up. When applied in anaphase, both drugs stopped or slowed poleward chromosome movements, usually reversibly. When applied near the end of prophase, both drugs often prevented one or more bivalents in the cell from attaching to the spindle. Attached bivalents behaved as in cells treated with drugs at later stages, as described above. Unattached bivalents in the same cells moved to poles or cytoplasm in early prometaphase, where they remained motionless; at anaphase they sometimes did not disjoin, but when they did disjoin the half-bivalents did not move, either in the continued presence of the drug or when CD was washed out, confirming that they were not atttached. When CD or LAT prevented all bivalents in the cell from attaching, spindles kept in the drug were invaded by granules at about the time of normal anaphase. Conversely, when CD was washed out during late prometaphase, chromosomes often attached to spindle fibres and later entered anaphase. As CD and LAT are different anti-actin drugs, but have the same effect on chromosome behaviour, the results implicate actin in early interactions of chromosomes with spindle fibres and in anaphase chromosome movements.
Similar content being viewed by others
References
Adames KA, Forer A (1996) Evidence for polewards forces on chromosome arms during anaphase. Cell Motil Cytoskel 34: 13–25.
Amankwah KS, De Boni U (1994) Ultrastructural localization of filamentous actin within neuronal interphase nuclei in situ. Exp Cell Res 210: 315–325.
Aubin JE, Osborn M, Weber K (1981) Inhibition of cytokinesis and altered contractile ring morphology induced by cytochalasins in synchronized PtK2 cells. Exp Cell Res 136: 63–79.
Auclair C, Messier P-E (1977) Revue des effets de la cytochalasine B sur la division, les mouvements, les échanges et al morphologie cellulaire. Rev Can Biol 36: 37–69.
Carter SB (1967) Effects of cytochalasins on mammalian cells. Nature 213: 261–264.
Cleary AL (1995) F-actin redistributions at the division site in living Tradescantia stomatal complexes as revealed by microinjection of rhodamine-phalloidin. Protoplasma 185: 152–165.
Cooper JA (1987) Effects of cytochalasin and phalloidin on actin. J Cell Biol 105: 1473–1478.
Coué M, Brenner SL, Spector I, Korn ED (1987) Inhibition of actin polymerization by latrunculin A. FEBS Lett 213: 316–318.
Czaban BB, Forer A (1992) Rhodamine-labelled phalloidin stains components in the chromosomal spindle fibres of crane-fly spermatocytes and Haemanthus endosperm. Biochem Cell Biol 70: 664–676.
Czaban BB, Forer A (1994) Rhodamine-phalloidin and antitubulin antibody staining of spindle fibres that were irradiated with an ultraviolet microbeam. Protoplasma 178: 18–27.
Dinis AM, Mesquita JF (1993) The F-actin distribution during microsporogenesis in Magnolia soulangeana Soul. (Magnoliaceae). Sex Plant Reproduct 6: 57–63.
Echeverri CJ, Paschal BM, Vaughan KT, Vallee RB (1996) Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J Cell Biol 132: 617–633.
Endow SA, Komma DJ (1996) Centrosome and spindle function of the Drosophila Ncd microtubule motor visualized in live embryos using Ncd-GFP fusion proteins. J Cell Sci 109: 2429–2442.
Forer A (1978) Chromosome movements during cell-division: possible involvement of actin filaments. In: Heath IB, ed. Nuclear Division in the Fungi. New York: Academic Press, pp 21–88.
Forer A (1982) Crane fly spermatocytes and spermatids: a system for studying cytoskeletal components. Methods Cell Biol 25: 227–252.
Forer A (1985) Does actin produce the force that moves a chromosome to the pole during anaphase? Can J Biochem Cell Biol 63: 585–598.
Forer A, Behnke O (1972a) An actin-like component in spermatocytes of a crane-fly (Nephrotoma suturalis Loew). I. The spindle. Chromosoma 39: 145–173.
Forer A, Behnke O (1972b) An actin-like component in spermatocytes of a crane-fly (Nephrotoma suturalis Loew). II. The cell cortex. Chromosoma 39: 175–190.
Forer A, Pickett-Heaps JD (1998) Checkpoint control in crane-fly spermatocytes: unattached chromosomes induced by cytochalasin D or latrunculin treatment do not prevent or delay the start of anaphase. Protoplasma 203: 100–111.
Forer A, Swedak J (1991) Practical experiences in using biological systems to monitor indoor air pollutants. In: Baird JC, Berglund B, Jackson WT, eds. Indoor Air Quality for People and Plants. Stockholm: Swedish Council for Building research, pp 129–158.
Forer A, Wilson PJ (1994) A model for chromosome movement during mitosis. Protoplasma 179: 95–105.
Forer A, Jackson WT, Engberg A (1979) Actin in spindles of Haemanthus katherinae endosperm. II. Distribution of actin in chromosomal spindle fibres, determined by analysis of serial sections. J Cell Science 37: 349–371.
Gaglio T, Saredi A, Bingham JB, Hasbani MJ, Gill SR, Schroer TA, Compton DA (1996) Opposing motor activities are required for the organization of the mammalian mitotic spindle pole. J Cell Biol 135: 399–414.
Inoué S, Salmon ED (1995) Force generation by microtubule assembly/disassembly in mitosis and related movements. Mol Biol Cell 6: 1619–1640.
Janicke MA, LaFountain JR Jr (1982) Chromosome segregation in crane-fly spermatocytes: cold treatment and cold recovery induce anaphase lag. Chromosoma 85: 619–631.
Janicke MA, LaFountain JR Jr (1984) Malorientation in half-bivalents at anaphase: analysis of autosomal laggards in untreated, cold-treated, and cold-recovering crane-fly spermatocytes. J Cell Biol 98: 859–869.
Janicke MA, LaFountain JR Jr (1986) Bivalent orientation and behavior in crane-fly spermatocytes recovering from cold exposure. Cell Motil Cytoskeleton 6: 492–501.
Kengen HMP, Eygensteyn J, van Amstel TNM (1995) F-actin in mitotic spindles of synchronized suspension culture cells of tobacco visualized by confocal laser scanning microscopy. Cell Biol Intern Rep 19: 585–592.
Kiehart DP, Mabuchi I, Inoué S (1982) Evidence that myosin does not contribute to force production in chromosome movement. J Cell Biol 94: 165–178.
Kubiak J, Paldi A, Weber M, Maro B (1991) Genetically identical parthenogenetic mouse embryos produced by inhibition of the first meiotic cleavage with cytochalasin D. J Cell Sci 111: 763–769.
LaFountain JR Jr, Janicke M, Balczon R, Rickards GK (1992) Cytochalasin induces abnormal anaphase in crane-fly spermatocytes and causes altered distribution of actin and centromeric antigens. Chromosoma 101: 425–441.
Lloyd CW, Traas JA (1988) The role of F-actin in determining the division plane of carrot suspension cells. Drug studies. Development 102: 211–221.
Mabuchi I, Okuno M (1977) The effect of myosin antibody on the division of starfish blastomeres. J Cell Biol 74: 251–263.
Milankov K, De Boni U (1993) Cytochemical localization of actin and myosin aggregates in interphase nuclei in situ. Exp Cell Res 209: 189–199.
Molè-Bajer J, Bajer AS (1988) Relation of F-actin organization to microtubules in drug treated Haemanthus mitosis. Protoplasma (Suppl. 1): 99–112.
Ohmori H, Toyama S, Toyama S (1992) Direct proof that the primary site of action of cytochalasin on cell motility processes is actin. J Cell Biol 116: 933–941.
Pickett-Heaps JD, Spurck TP (1982) Studies on kinetochore function in mitosis. I. The effects of colchicine and cytochalasin on mitosis in the diatom Hantzschia amphioxys. Eur J Cell Biol 28: 77–82.
Pickett-Heaps JD, Forer A, Spurck T (1996) Rethinking anaphase: where “PacMan” fails and why a role for the spindle matrix is likely. Protaplasma 192: 1–10.
Pickett-Heaps JD, Forer A, Spurck T (1997) The traction fibre: toward a “tensegral” model of the spindle. Cell Motil Cytoskel 37: 1–6.
Rungger E, Rungger-Brändle E, Chaponnier C, Gabbiani G (1979) Intranuclear injection of anti-actin antibodies into Xenopus oocytes blocks chromosome condensation. Nature 282: 320–321.
Sampath P, Pollard TD (1991) The effects of cytochalasin, phalloidin and pH on the elongation of actin filaments. Biochemistry 30: 1973–1980.
Sampson K, Pickett-Heaps JD, Forer A (1996) Cytochalasin D blocks chromosomal attachment to the spindle in the green alga Oedogonium. Protoplasma 192: 130–144.
Schaap CJ, Forer A (1979) Temperature effects on anaphase chromosome movement in the spermatocytes of two species of crane flies (Nephrotoma suturalis Loew and Nephrotoma ferruginea Fabricius). J Cell Sci 39: 29–52.
Schafer DA, Gill SR, Cooper JA, Heuser JE, Schroer TA (1994) Ultrastructural analysis of the dynactin complex: an actin-related protein is a component of a filament that resembles F-actin. J Cell Biol 126: 403–412.
Schatten G, Schatten H, Spector I, Cline C, Paweletz N, Simerly C, Petzelt C (1986) Latrunculin inhibits the microfilament-mediated processes during fertilization, cleavage and early development in sea urchins and mice. Exp Cell Res 166: 191–208.
Schibler MJ, Pickett-Heaps JD (1980) Mitosis in Oedogonium: spindle microfilaments and the origin of the kinetochore fibre. Eur J Cell Biol 22: 687–698.
Schmit A-C, Lambert A-M (1987) Characterization and dynamics of cytoplasmic F-actin in higher plant endosperm cells during interphase, mitosis, and cytokinesis. J Cell Biol 105: 2157–2166.
Schmit A-C, Lambert A-M (1988) Plant actin filament and microtubule interactions during anaphase-telophase transition: effects of antogonist drugs. Biol Cell 64: 309–319.
Schmit A-C, Lambert A-M (1990) Microinjected fluorescent phalloidin in vivo reveals the F-actin dynamics and assembly in higher plant mitotic cells. Plant Cell 2: 129–138.
Schroer TA, Bingham JB, Gill SR (1996) Actin-related protein 1 and cytoplasmic dynein-based motility–what's the connection? Trends Cell Biol 6: 212–215.
Seagull RW, Falconer MM, Weerdenburg CA (1987) Microfilaments: dynamic arrays in higher plant cells. J Cell Biol 104: 995–1004.
Sheldon JM, Hawes C (1988) The actin cytoskeleton during male meiosis in Lilium. Cell Biol Internatl Rep 12: 471–476.
Snyder JA, Cohen L (1995) Cytochalasin J affects chromosome congression and spindle microtubule organization in PtK1 cells. Cell Motil Cytoskel 32: 245–257.
Spector I, Schochet NR, Kashman Y, Groweiss A (1983) Latrunculins: novel marine toxins that disrupt microfilament organization in cultured cells. Science 219: 493–495.
Spector I, Shochet NR, Blasberger D, Kashman Y (1989) Latrunculins-novel marine macrolides that disrupt microfilament organization and affect cell growth: I. Comparison with cytochalasin D. Cell Motil Cytoskel 13: 127–144.
Spurck T, Forer A, Pickett-Heaps J (1997) Ultraviolet microbeam irradiations of fibroblast and spermatocyte spindles suggest that forces act on the kinetochore fibre and are not generated by its disassembly. Cell Motil Cytoskel 36: 136–148.
Traas JA, Doonan JH, Rawlins DJ, Shaw PJ, Watts J, Lloyd CW (1987) An actin network is present in the cytoplasm throughout the cell cycle of carrot cells and associates with the dividing nucleus. J Cell Biol 105: 387–395.
Traas JA, Burgain S, De Vaulx RD (1989) The organization of the cytoskeleton during meiosis in eggplant (Solanum melongena (L.)): microtubules and F-actin are both necessary for coordinated meiotic division. J Cell Sci 92: 541–550.
Urbanik E, Ware BR (1989) Actin filament capping and cleaving activity of cytochalasins B, D, E, and H. Arch Biochem Biophys 269: 181–187.
Valdivia MM, Brinkley BR (1984) Biochemical studies of the kinetochore/centromere of mammalian chromosomes. In: Borisy GG, Cleveland DW, Murphy DB, eds. Molecular Biology of the Cytoskeleton. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, pp 79–86.
Vallee RB, Sheetz MP (1996) Targetting of motor proteins. Science 271: 1539–1544.
Van Lammeren AAM, Bednara J, Willemse MTM (1989) Organization of the actin cytoskeleton during pollen development in Gasteria verrucosa (Mill.) H. Duval visualized with rhodamine-phalloidin. Planta 178: 531–539.
Waterman-Storer CM, Salmon ED (1997) Actomyosin-based retrograde flow of microtubules in the lamella of migrating epithelial cells influences microtubule dynamic instability and turnover and is associated with microtubule breakage and treadmilling. J Cell Biol 139: 417–434.
Wilson P, Forer A (1989) The behaviour of microtubules in chromosomal spindle fibres irradiated singly or doubly with ultraviolet light. J Cell Sci 94: 625–634.
Wilson PJ, Forer A, Leggiadro C (1994) Evidence that kinetochore microtubules in crane-fly spermatocytes disassemble during anaphase primarily at the poleward end. J Cell Sci 107: 3015–3027.
Wrench GA, Snyder JA (1997) Cytochalasin J treatment signi ficantly alters mitotic spindle microtubule organization and kinetochore structure in PtK1 cells. Cell Motil Cytoskel 36: 112–124.
Yu H-G, Hiatt EN, Chen A, Sweeney M, Dawe RK (1997) Neocentromere-mediated chromosome movement in maize. J Cell Biol 139: 831–840.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Forer, A., Pickett-Heaps, J.D. Cytochalasin D and Latrunculin Affect Chromosome Behaviour During Meiosis in Crane-fly Spermatocytes. Chromosome Res 6, 533–550 (1998). https://doi.org/10.1023/A:1009224322399
Issue Date:
DOI: https://doi.org/10.1023/A:1009224322399