Abstract
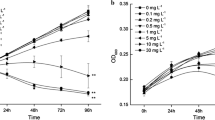
Short-term toxicity tests using photosynthesis (incorporation of 14C) as a test parameter were performed in order to compare the sensitivities of three marine microalgal communities (phytoplankton, periphyton and epipsammon) to two herbicides, paraquat and simazine. Thirty minutes of pre-exposure to simazine were sufficient to obtain the full effect in all communities, while for paraquat 4 h was required. The bioavailability of paraquat and simazine was not limited by adsorption to sediment in the epipsammon samples. Simazine was more toxic than paraquat for the three communities at similar concentrations. Phytoplankton was slightly more sensitive for both herbicides (EC50 ranges of 9--23 mu m for paraquat and 0.37--0.99 mu m for simazine) than periphyton and epipsammon. These attached communities exhibited different results for each toxicant, periphyton being more sensitive to paraquat (EC50 range 9--21 mu m) and epipsammon to simazine (EC50 range 0.44--1.17 mu m). The three communities presented EC ranges comparable to those found in single species tests, suggesting that different levels of biological organization can exhibit a similar sensibility to toxicants, thus indicating that natural communities are suitable for use in these kinds of toxicity tests
Similar content being viewed by others
References
Blanck, H. (1983) On the impact of long-chained aliphatic amines on photosynthesis and algal growth in ecotoxicological test systems. PhD thesis, Department of Plant Physiology, University of Göteborg.
Blanck, H. (1985) A simple, community level, ecotoxicological test system using samples of periphyton. Hydrobiologia 124, 251–61.
Blanck, H. and Wängberg, S-Å. (1988) Validity of an ecotoxicological test system: short-term and long-term effects of arsenate on marine periphyton communities in laboratory systems. Can. J. Fish. Aquatic Sci. 45, 1807–15.
Blanck, H., Wallin, G. and Wängberg, S-Å. (1984) Species dependent variation in algal sensitivity to chemical compounds. Ecotoxicol. Environ. Safety 8, 339–51.
Bozeman, J., Koopman, B. and Bitton, B. (1989) Toxicity testing using mobilized algae. Aquatic Toxicol. 14, 345–52.
Cairns, J. (1986) The myth of the most sensitive species. BioScience 36, 670–2.
Cairns, J. and Pratt, J.R. (1989) The scientific basis of bioassays. Hydrobiologia 188/189, 5–20.
Clements, W.H. and Kiffney, P.M. (1994) Assessing contaminant effects at higher levels of biological organization. Environ. Toxicol. Chem. 13, 357–9.
Couture, P., Thellen, C. and Thompson, P. (1989) Phytoplankton recovery responses at the population and community levels in a hazard and risk assessment study. Hydrobiologia 188/189, 269–76.
Cserháti, T. (1993) Interaction of diquat and paraquat with humic acid and the influence of salt concentration and pH on the strength on interaction. Fresenius J. Anal. Chem. 345, 541–4.
Dahl, B. and Blanck, H. (1996) The use of sand-living microalgal communities (epipsammon) in ecotoxicological testing. Mar. Ecol.-Prog. Ser. 144, 163–73.
DeNoyelles, F., Kettle, W.D. and Sinn, D.E. (1982) The responses of plankton communities in experimental ponds to atrazine, the most heavily used pesticide in the United States. Ecology 63, 1285–93.
Elliot, J.M. (1983) Some Methods for the Statistical Analysis of Samples of Benthic Invertebrates. Scientific Publication No. 25, Freshwater Biological Association.
Erickson, L.E. and Lee, K.H. (1989) Degradation of atrazine and related s-triazines. Crit. Rev. Environ. Control 19, 1–13.
Fedtke, C. (1982) Biochemistry and Physiology of Herbicide Action. New York: Springer-Verlag.
Francois, D.L. and Robinson, G.C. (1990) Indices of triazine toxicity in Chlamydomonas geitleri. Aquatic Toxicol. 16, 205–28.
Goldsborough, L. and Robinson, G.C. (1986) Changes in periphytic algal community structure as a consequence of short herbicide exposures. Hydrobiologia 139, 177–92.
Goldsborough, L. and Robinson, G.C. (1988) Functional responses of freshwater periphyton to short simazine exposures. Verh. Int. Verein. Limnol. 23, 1586–93.
Gurney, S.E. and Robinson, G.C. (1989) The influence of two triazine herbicides on the productivity, biomass and community composition of freshwater marsh periphyton. Aquatic Bot. 36, 1–22.
Hakansson, L. and Jansson, M. (1993) Principles of Lake Sedimentology. Berlin: Springer-Verlag.
Hansson, O. and Wydrzynski, T. (1990) Current perceptions of photosystem II. Photosystem Res. 23, 131–62.
Hiraprandit, H. and Foy, C.L. (1992) Effect of four triazine herbicides on growth of nontarget green algae. Weed Sci. 40, 134–42.
Ibrahim, E.A. (1990) The influence of the herbicide paraquat “Gramaxon” on growth and metabolic activity of three chlorophytes. Water, Air Soil Pollut. 51, 89–93.
Kookana, R.S. and Aylmore, A.G. (1993) Retention and release of diquat and paraquat herbicides in soil. Aust. J. Soil. Res. 31, 97–109.
Lagoutte, B. and Mathis, P. (1989) The photosystem I reaction center: structure and photochemistry. Photochem. Photobiol. 49, 833–44.
Landner, L., Blanck, H., Heyman, U., Lundgren, A., Notini, M., Rosemarin, A. and Sundelin, A. (1989) Community testing, microcosm and mesocosm experiments: ecotoxicological tools with high ecological realism. In Chemicals in the Aquatic Environment — Advanced Hazard Assessment, p. 216–54 (L. Landner, ed). Berlin: Springer-Verlag.
Lanno, R.P., Hickie, H.E. and Dixon, D.G. (1989) Feeding and nutritional considerations in aquatic toxicology. Hydrobiologia 188/189, 525–31.
Mauck, W.L., Mayer, F.L. and Holz, D.D. (1976) Simazine residue dynamics in small ponds. Bull. Environ. Contam. Toxicol. 16, 1–8.
Munawar, M., Munawar, I.F. and Leppard, G.G. (1989) Early warning assays: an overview of toxicity testing with phytoplankton in the North American Great Lakes. Hydrobiologia 188/189, 237–46.
Naqvi, S.M., Leung, T.S. and Naqvi, N.Z. (1981) Toxicities of paraquat and metribuzin (Sencor) herbicides to the freshwater copepods, Eucyclops agilis and Diaptomus mississippiensis. Environ. Pollut. (Ser A) 26, 275–80.
Nicholls, P.H., Briggs, G.C. and Evans, A.A. (1984) The influence of water solubility on the movement and degradation of simazine in fallow soil. Weed Res. 24, 37–49.
O'Neill, R.V., Gardner, R.H., Barnthouse, L.W., Suter, G.W., Hildebrand, S.G. and Gehrs, C.W. (1982) Ecosystem risk analysis: a new methodology. Environ. Toxicol. Chem. 1, 167–77.
Rochaix, J-D. and Erickson, J. (1988) Function and assembly of photosystem II: genetic and molecular analysis. TIBS 13, 56–9.
Smith, L.L. (1988) The toxicity of paraquat. Adv. Drug. React. Ac. Poison Rev. 1, 1–17.
Svansson, A. (1984) Hydrography of Gullmar Fjord. Sweden: Institute of Hydrography Research (23), Fishery Board of Sweden.
Tubbing, D.M.J., Admiraal, W., Blanck, H., Sabater, S. and Guasch, H. (1996) Can periphyton communities be used to detect pollution with chemicals? In Use of Algae to Monitoring Rivers II, pp. 59–62 (B. Whitton, ed). Innsbruck: Institut für Botanik, Universität Innsbruck.
Turbak, S.C., Olson, S.B. and McFeters, G.A. (1986) Comparison of algal assay systems for detecting waterborne herbicides and metals. Water. Res. 20, 91–6.
Wängberg, S.Å. (1989) Arsenate tolerance in periphyton and phytoplankton, PhD thesis. Göteborg: Department of Plant Physiology, University of Göteborg.
Weber, A. (1981) An uncomplicated screening test to evaluate toxicity of environmentally hazardous compounds in water. Environ. Technol. Lett. 2, 323–8.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bonilla, S., Conde, D. & Blanck, H. The photosynthetic responses of marine phytoplankton, periphyton and epipsammon to the herbicides paraquat and simazine. Ecotoxicology 7, 99–105 (1998). https://doi.org/10.1023/A:1008867920179
Issue Date:
DOI: https://doi.org/10.1023/A:1008867920179