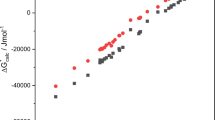
Abstract
To extend the successful application of Hammett equations, previously used to predict equilibrium and rates of physico-chemical reactions with electronic and steric parameters, to the realm of biology and biochemistry, a parameter that measures hydrophobicity is required. The partition coefficient of a solute between octanol and water, expressed in log terms to put it on the same free-energy basis as the classic Hammett parameters, has been shown to be widely applicable. It is directly involved in passive transport through membranes, in binding to proteins, and in specific binding at active sites in enzymes. Methods of calculating logP(octanol) that reflect the solvation forces involved, can be useful in elucidating unusual solute conformations that may be preferred in a non-polar environment.
Similar content being viewed by others
References
Hansch, C., Hoekman, D. and Gao, H., Chem. Rev., 96 (1996) 1045.
Hansch, C., Gao, H. and Hoekman, D, In Devillers, J. (Ed.) Comparative QSAR, Taylor and Francis, London, 1998, pp. 285–368.
C-QSAR and CLOGP programs, BioByte Corporation, Claremont, CA.
Hansch, C. and Klein, T.E., Acc. Chem. Res., 19 (1986) 392.
Selassie, C.D., Li, R.-L., Poe, M. and Hansch, C., J. Med. Chem., 34 (1991) 46.
Selassie, D.C., de Soyza, T.V., Rosario, M., Gao, H. and Hansch, C., Chem.-Biol. Interact., 113 (1998) 175.
Hansch, C. and Gao, H., Chem. Rev., 97 (1997) 2995.
Rawn, J.D., In Biochemistry, Niel Patterson Publ., Burlington, NC, 1989, Chapter 5.2.a.
'Protein-Fold’ program, Tripos, St. Louis, MO.
El Tayar, N., Mark, A.E., Vallat, P., Brunne, R.M., Testa, B. and van Gunsteren, W.F., J. Med. Chem., 36 (1993) 3757.
DeBolt, S.E. and Kollman, P.A., J. Am. Chem. Soc., 117 (1995) 5316.
Reynolds, C.H., J. Chem. Inf. Comput. Sci., 35 (1995) 738.
Reynolds, C.H., Chem. Tech., Nov. (1998) 28.
Palm, K., Luthman, K., Ungell, A.-L., Standlund, G., Beigi, F., Lundahl, P. and Artursson, P., J. Med. Chem., 41 (1998) 5382.
Steinmetz, W., Bersch, R., Towson, J. and Pesiri, D., J. Med. Chem., 35 (1992) 4842.
Stephenson, G., Stowell, J., Toma, P., Pfeiffer, R. and Byrn, S., J. Pharm. Sci., 86 (1997) 1239.
CambridgeSoft Corp., Cambridge, MA.
Tripos, SYBYL and CoMFA, Tripos, St. Louis, MO.
Kellogg, G.E., Semus, S.F. and Abraham, D.J., J. Comput.-Aided Mol. Design, 5 (1991) 545.
Testa, B., Carrupt, P.-A., Gaillard, P., Billois, F. and Weber, P., Pharm. Res., 13 (1996) 335.
Marcel, C., Boss, G., van de Waterbeemd, H. and Testa, B., Eur. J. Med. Chem., 20 (1985) 459.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Leo, A.J., Hansch, C. Role of hydrophobic effects in mechanistic QSAR. Perspectives in Drug Discovery and Design 17, 1–25 (1999). https://doi.org/10.1023/A:1008762321231
Issue Date:
DOI: https://doi.org/10.1023/A:1008762321231