Abstract
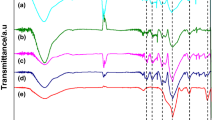
Highly proton-conductive elastic composites have been successfully prepared from H3PO4-doped silica gel and a styrene-ethylene-butylene-styrene (SEBS) block elastic copolymer. Ionic conductivities of the composites depended on the concentration of H3PO4 and the heat treatment temperature of the H3PO4-doped silica gel. It was found that H3PO4 added is present mainly as free orthophosphoric acid in the silica gel. The composite composed of H3PO4-doped silica gel with a molar ratio of H3PO4/SiO2 = 0.5 heat-treated at temperatures below 200°C and SEBS elastomer in 5 mass% showed a high conductivity of 10−5 S cm−1 at 25°C in an dry N2 atmosphere. The water adsorption during a storage in 25% relative humidity at room temperature for 1 day enhanced the ionic conductivities of composites by about one order of magnitude. Lower conductivities obtained in the composite with the H3PO4-doped silica gel heat-treated at 250°C for 1 h were due to the formation of crystalline Si3(PO4)4. The temperature dependence of conductivity of the composites was the Vogel-Tamman-Fulcher type, indicating that proton was transferred through a liquidlike phase formed in micropores of the H3PO4-doped silica gels. The temperature dependence of the modulus of the composite was similar to that of the SEBS elastomer. The thermoplastically deforming temperature of the composite was around 100°C, which was higher by 30°C than that of the SEBS elastomer.
Similar content being viewed by others
REFERENCES
H. Iwahara, H. Uchida, and S. Tanaka, Solid State Ionics 9/10, 1021 (1983).
M. Kotama, K. Nakanishi, H. Hosono, and Y. Abe, J. Electrochem. Soc. 138, 2928 (1991).
Ph. Colomban (Ed.), Proton Conductors (Cambridge University Press, Cambridge, 1992).
M. Tatsumisago and T. Minami, J. Am. Ceram. Soc. 72, 484 (1989).
M. Tatsumisago, Y. Sakai, H. Honjo, and T. Minami, J. Ceram. Soc. Japan 103, 189 (1995).
M. Tatsumisago, H. Honjo, Y. Sakai, and T. Minami, Solid State Ionics 74, 105 (1994).
A. Matsuda, H. Honjo, M. Tatsumisago, and T. Minami, Chem. Lett. 1189 (1998).
H. Honjo, M. Tatsumisago, and T. Minami, J. Mater. Sci. 14, 783 (1995).
A. Matsuda, H. Honjo, M. Tatsumisago, and T. Minami, Solid State Ionics 113–115, 91 (1998).
A. Matsuda, H. Honjo, K. Hirata, M. Tatsumisago, and T. Minami, J. Power Sources 77, 12 (1999).
JCPDS #18-1167, #18-1168 (International Centre for Diffraction Date, 1987).
C. Fernandes-Lorenzo, L. Esquivias, P. Barboux, J. Maquet, and F. Taulelle, J. Non-Cryst. Solids 176, 189 (1994).
F.M. Gray, J.R. MacCallum, C.A. Vincent, and J.R.M Giles, Macromolecules 21, 392 (1988).
M. Nogami, R. Nagao, K. Makita, and Y. Abe, Appl. Phys. Lett. 71, 1 (1997).
M. Nogami, K. Miyamura, and Y. Abe, J. Electrochem. Soc. 144, 121 (1997).
K.D. Berlin, G.M. Blackburn, D.E.C. Corbridge, and D.M. Hellwege, Topics in Phosphorus Chemictry, ed. by M. Grayson and E. Griffith, Vol. 6 (A Wiley-Interscience Publication, New York, 1969) p. 269.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hirata, K., Matsuda, A., Hirata, T. et al. Preparation and Characterization of Highly Proton-Conductive Composites Composed of Phosphoric Acid-Doped Silica Gel and Styrene-Ethylene-Butylene-Styrene Elastomer. Journal of Sol-Gel Science and Technology 17, 61–69 (2000). https://doi.org/10.1023/A:1008713122220
Issue Date:
DOI: https://doi.org/10.1023/A:1008713122220