Abstract
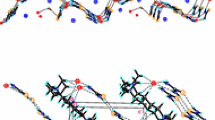
New inclusion complexes(C2H5)4N+HCO2-·(NH2)2CS·H2O (1),[(C2H5)4N]2+[(HCO2)2H]-(HCO2-)·2(NH2)2CS(2), (n-C3H7)4N+HCO2-·3(NH2)2CS·H2O (3)and (n-C4H9)4N+[(HCO2)2H]-·2(NH2)2CS(4) have been prepared and characterized by X-ray crystallography. Crystal data, MoKα radiation: 1, space group P21/c, a = 7.199(2), b = 16.851(2),c = 13.044(2) Å, β = 100.13(2)°, Z = 4, and RF = 0.065 for 1011 observed data; 2, space group Pca21, a = 25.803(5), b = 7.190(2), c = 17.394(2) Å, Z = 4, and RF= 0.073 for 1515 observed data; 3, space group P21/n, a = 8.533(2), b = 9.423(5), c = 33.517(7) Å, β = 90.44(2)°, Z = 4, and RF = 0.052 for 2521 observed data; 4, space group Pbca, a = 17.389(3), b = 16.622(2),c = 20.199(3) Å, Z = 8, and RF = 0.056for 1910 observed data. In both 1 and 2 the tetraethylammonium ions are sandwiched between puckered layers, which are constructed by the cross-linkage of a parallel arrangement of infinite chains. In 1 each chain is composed of twisted(thiourea–formate)2 tetramers bridged by water molecules, whereas in 2 it comprises an alternate arrangement of thiourea dimers and protonated formate trimers each formed by the linkage of a hydrogen diformate ion, [(HCO2)2H]-, to a formate ion via} a C–-H·sO hydrogen bond. In compound 3 two independent thiourea molecules are used to construct a hydrogen-bonded puckered layer normal to thec axis, whereas the remaining thiourea molecule, together with the formate ion and water molecule, generate another puckered layer that is parallel to the first one. Hydrogen bonding between these two types of layers gives rise to a network containing channels running parallel to the [100] direction, and the cations are stacked regularly within each column. In the crystal structure of 4, the thiourea molecules form hydrogen-bonded zigzag ribbons running parallel to the b axis, which are cross-linked by the dimeric formate moieties [(HCO2)2H]- to form a puckered layer, and the(n-C4H9)N+ cations occupy the space between adjacent layers.
Similar content being viewed by others
References
K. Takemota and N. Sonoda: in Inclusion Compounds(Eds. J.L. Atwood, J.E.D. Davies, and D.D. MacNicol), Vol. II, pp. 47–67. Academic Press, London (1984).
L.C. Fetterly: in Non-stoichiometric Compounds(Ed. L. Mandelcorn), pp. 491–567. Academic Press, New York (1964).
K.D.M. Harris and J.M. Thomas: J. Chem. Soc., Faraday Trans. 86, 2985 (1990); K.D.M. Harris, S.P. Smart, and M.D. Hollingsworth: ibid. 87, 3423 (1991).
K.D.M. Harris: Chem. Brit. 29, 132 (1993).
H.-U. Lenné: Acta Crystallogr. 7, 1 (1954).
E. Hough and D.G. Nicholson: J. Chem. Soc., Dalton Trans. 15 (1978).
K.D.M. Harris and J.M. Thomas: J. Chem. Soc., Faraday Trans. 86, 1095 (1990).
A.R. George and K.D.M. Harris: J. Mol. Graphic. 13, 138 (1995).
T.C.W. Mak and G.D. Zhou: Crystallography in Modern Chemistry, p. 180, Wiley, New York (1992).
P.A. Schofield, K.D.M. Harris, I.J. Shannon, and A.J.O. Rennie: J. Chem. Soc., Chem. Commun. 1293 (1993).
A.G. Anderson, J.C. Calabrese, W. Tam, and I.D. Williams: Chem. Phys. Lett. 134, 392 (1987).
A.E. Tonelli: Comput. Polym. Sci. 2(2–3), 80 (1992).
R.K. McMullan, T.C.W. Mak, and G.A. Jeffrey: J. Chem. Phys. 44, 2338 (1966).
W.J. McLean and G.A. Jeffrey: J. Chem. Phys. 47, 414 (1967).
W.J. McLean and G.A. Jeffrey: J. Chem. Phys. 49, 4556 (1968).
T.C.W. Mak: J. Incl. Phenom. 4, 273 (1986).
M. Wiebcke, C.C. Freyhardt, J. Felsche, and G. Engelhardt: Z. Naturforsch., B: Chem. Sci. 48, 978 (1993).
G.A. Jeffrey and W. Saenger: Hydrogen Bonding in Biological Structures, Springer Verlag, Berlin (1991).
T. Steiner and W. Saenger: J. Chem. Soc., Chem. Commun. 2087 (1995).
K. Gessler, N. Krauss, T. Steiner, C. Betzel, A. Sarko, and W. Saenger: J. Am. Chem. Soc. 117, 11397 (1995).
F. Holtzberg, B. Post, and I. Fankuchen: Acta Crystallogr. 6, 127 (1953).
I. Nahringbauer: Acta Crystallogr., Sect. B 34, 315 (1978).
Z. Berkovitch-Yellin and L. Leiserowitz: J. Am. Chem. Soc. 104, 4052 (1982).
T. Neuheuser, B.A. Hess, C. Reutel, and E. Weber: J. Phys. Chem. 98(26), 6459 (1994).
T.C.W. Mak: J. Incl. Phenom. 8, 199 (1990). Part I of this series.
T.C.W. Mak, W.H. Yip, and Q. Li: J. Am. Chem. Soc. 117, 11995 (1995).
Q. Li and T.C.W. Mak: Acta Crystallogr., Sect. B 52, 989 (1996). Part III of this series.
Q. Li and T.C.W. Mak: J. Incl. Phenom. 20, 73 (1995). Part II of this series.
R.T. Morrison and R.N. Boyd: Organic Chemistry, 6th ed., p. 854, Prentice-Hall, London (1992).
R.A. Sparks: in Crystallographic Computing Techniques(Ed. F.R. Ahmed), p. 452. Munksgaard, Copenhagen (1976).
G. Kopfmann and R. Huber: Acta Crystallogr., Sect. A 24, 348 (1968).
G.M. Sheldrick: in Computational Crystallography(Ed. D. Sayre), Oxford University Press, New York (1982), pp. 506–514.
International Tables for X-ray Crystallography, Vol. IV, Kynoch Press, Birmingham (1974) (Distrib.: Kluwer Academic Publishers, Dordrecht), pp. 55, 99, 149.
Q. Li and T.C.W. Mak: J. Incl. Phenom.Part V of this series (accepted).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
LI, Q., MAK, T.C. Inclusion Compounds of Thiourea and Peralkylated Ammonium Salts. Part IV. Hydrogen-Bonded Host Lattices Built of Thiourea and Formate Ions. Journal of Inclusion Phenomena 27, 319–340 (1997). https://doi.org/10.1023/A:1007928123267
Issue Date:
DOI: https://doi.org/10.1023/A:1007928123267