Abstract
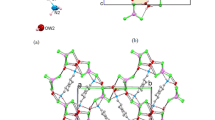
Two new inclusion compounds, namely 3(CH3)3N+(CH2)3N+(CH3)3·6Br–·8(NH2)2CS·H2O (1) and (CH3)3N+(CH2)4N+(CH3)3·2Br–·2(NH2)2CS (2), have been prepared and characterized by X-ray crystallography. In the crystal structure of 1, uni-directional double channels with peanut-shaped cross-section running paralleling to the a axis are generated by N–H···S and N–H···Br hydrogen bonds between thiourea molecules and bromide anions, and trimethylenebis(trimethylammonium) dications are arranged separately in each hemisphere of the double channel. In 2, tetramers comprising pairs of thiourea molecules and bromide anions by N–H···S and N–H···Br hydrogen bonds are joined with the methyl groups of tetramethylenebis(trimethylammonium) dications to generate similar quasi-rectangle channels. The present study shows that new anionic host lattices can be constructed from thiourea molecules and bromide anions as building blocks, which readily adopt different topologies for the accommodation of bis-quaternary ammonium dications of various sizes. Hydrogen bonding constitutes a principal driving force that dominates the crystal packing of the two compounds, in which weak hydrogen bonds are of particular significance in the construction.
Graphical Abstract
In the crystal structure of 1, uni-directional double channels with peanut-shaped cross-section running paralleling to the a axis are generated by N–H···S and N–H···Br hydrogen bonds between thiourea molecules and bromide anions, and trimethylenebis(trimethylammonium) dications are arranged separately in each hemisphere of the double channel. In 2, tetramers comprising pairs of thiourea molecules and bromide anions by N–H···S and N–H···Br hydrogen bonds are joined with the methyl groups of tetramethylenebis(trimethylammonium) dications to generate similar quasi-rectangle channels.







Similar content being viewed by others
References
Burrows AD (2004) In: Atwood JL (ed) Encyclopedia of Supramolecular Chemistry. Taylor & Francis Group, New York
Han J, Zhao L, Yau CW, Mak TCW (2009) Cryst Growth Des 9:308
Chakrabarty R, Mukherjee PS, Stang PJ (2011) Chem Rev 111:6810
Desiraju GR (2007) Angew Chem Int Ed 46:8342
Paik WC, Shin CH, Lee JM, Ahn BJ, Hong SB (2001) J Phys Chem B 105:9994
Mak TCW, Li Q (1998) In: Hargittai M (ed) Advances in Molecular Structure Research. JAI Press, Greenwich
Mak TCW, Lam CK, Han J, Li Q, Xue F (2010) In: Vittal J (ed) Organic Crystal Engineering-Frontiers in Crystal Engineering. Wiley, Chichester
Yang Y, Yin B, Li Q, Englert U (2009) J Mol Struct 920:401
Yang YX, Li K, Li Q (2010) J Mol Struct 969:83
Carpenter P, Lindenbaum S (1977) J Solut Chem 6:581
SMART APEX software (5.624) for SMART APEX detector, Bruker Axs Inc. Madison. Wisconsin, USA
SAINT software (6.02) for SMART APEX detector, Bruker Axs Inc. Madison. Wisconsin, USA
Sheldrick GM (1996) SADABS. Program for empirical absorption correction of area detector data. University of Gottingen, Gottingen
Sheldrick GM (2008) Acta Cryst A 64:112
Spek AL (2003) J Appl Cryst 36:7
Li Q, Xue F, Mak TCW (1999) Inorg Chem 38:4142
Thackeray MM, Coetzer J (1976) Acta Cryst B 32:2966
Cornelissen JP, Muller E, Vaassens PHS, Haasnoot JG, Reedijk J, Cassoux P (1992) Inorg Chem 31:2241
Neve F, Crispini A (2001) Cryst Growth Des 1:387
Desiraju GR, Das D (2006) Chem Asian J 1:231
Potrzebowski MJ, Michalska M, Kozioł AE, Kazùmierski S, Lis T, Pluskowski J, Ciesielski W (1998) J Org Chem 63:4209
Acknowledgments
Support from the NSFC (Grant Nos. 20571012 and 20871019) are gratefully acknowledged. We thank the Science Technology Foundation of Guizhou (No. [2009] 2266) and the Doctoral Foundation of Guizhou Normal University for supporting this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, Y., Li, K., Yang, YX. et al. Crystalline Inclusion Compounds: Thiourea and Bis-quaternary Ammonium Salts. J Chem Crystallogr 45, 120–127 (2015). https://doi.org/10.1007/s10870-015-0571-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-015-0571-5