Abstract
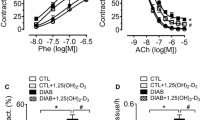
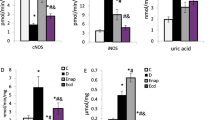
Oxygen-derived free radicals are believed to be involved in diabetes-induced vascular complications. The role of oxygen radicals in endothelial dysfunction in diabetes is not known with certainty. In this study we tested whether inhibition of lipid peroxidation using the potent inhibitor U74389F, a 21-aminosteroid also known as lazaroid, could prevent endothelial dysfunction in diabetes. Lewis strain rats were made diabetic by intravenous injection of streptozotocin. A subgroup of diabetic animals received daily oral doses of 10 mg/kg U74389F at 72 hours post streptozotocin and throughout the 8-week duration of diabetes. Thoracic aortas were isolated and suspended in isolated tissue baths and contracted with norepinephrine. Relaxation due to the endothelium-dependent vasodilator, acetylcholine, was impaired in diabetic aorta while relaxation due to A23187 and nitroglycerin was unaltered. Chronic treatment of diabetic animals with U74389F normalized the increase in plasma lipid peroxides as assessed by thiobarbituric acid–reactive substances but did not alter serum insulin levels, blood glucose concentration, nor total glycosylated hemoglobin. Increases in aortic catalase activity resulting from diabetes was not altered by U74389F. Despite reductions in lipid peroxides, U74389F did not prevent the diabetes-induced impairment in endothelium-dependent relaxation caused by acetylcholine. These data suggest that other pathways that are antecedent to lipid peroxidation may be responsible for endothelial dysfunction in diabetes.
Similar content being viewed by others
References
Oberley LW. Free radicals and diabetes. Free Radical Biol Med 1988;5:113–124.
Wolff SP. Diabetes mellitus and free radicals. Free radicals, transition metals and oxidative stress in the aetiology of diabetes mellitus and complications. Br Med J 1993;49:642–652.
Roza AM, Slakey DP, Pieper GM, et al. Hydroxyethyl starch deferoxamine, a novel iron chelator, delays diabetes in BB rats. J Lab Clin Med 1994;123:556–560.
Drash AL, Rudert WA, Borquaye S, Wang R. Effect of probucol on development of diabetes mellitus in BB rats. Am J Cardiol 1988;62:27B–30B.
Rabinovitch A, Suarez WL, Power RF. Lazaroid antioxidant reduces incidence of diabetes and insulitis in nonbese diabetic mice. J Lab Clin Med 1993;121:603–607.
Rösen P, Zink S, Tschöpe D. Vascular damage due to oxidative stress: A pathogenetic concept for diabetic macro-and microangiopathy? In: Weber B, Burger W, Danne T, eds. Structural and Functional Abnormalities in Subclinical Diabetic Angiopathy. Pediatr Adolesc Endocrinol 1992;22:23–31.
Sato Y, Hotta N, Sakamoto N, Matsuoka S, Oshishi N, Yagi K. Lipid peroxide levels of plasma of diabetic patients. Biochem Med 1979;21:104–107.
Armstrong D, Al-Awadi F. Lipid peroxidation and retinopathy in streptozotocin-induced diabetes. Free Radical Biol Med 1991;11:433–436.
Gallou G, Ruelland A, Legras B, Maugendre D, Allannic A, Clourec L. Plasma malondialdehyde in type 1 and type 2 diabetic patients. Clin Chim Acta 1993;214:227–234.
Pieper, GM, Gross GJ. Oxygen free radicals abolish endothelium-dependent relaxation in diabetic rat aorta. Am J Physiol 1988;255 (Heart Circ Physiol 24):H825-H833.
Oyama Y, Kawasaki H, Hattori Y, Kanno M. Attenuation of endothelium-dependent relaxation in aorta from diabetic rats. Eur J Pharmacol 1986;131:75–78.
Tesfamariam B, Jakubowski JA, Cohen RA. Contraction of diabetic rabbit aorta caused by endothelium-derived PGH2-TxA2. Am J Physiol 1989;257 (Heart Circ Physiol 26):H1327-H1333.
Kamata K, Miyata N, Kasuya Y. Impairment of endothelium-dependent relaxation and changes on levels of cyclic GMP in aorta from streptozotocin-induced diabetic rats. Br J Pharmacol 1989;97:614–618.
Mayhan WG. Impairment of endothelium-dependent dilatation of the basilar artery during diabetes mellitus. Brain Res 1992;580:297–302.
Johnstone MT, Creager SJ, Scales KM, Cusco JA, Lee BK, Creager MA. Impaired endothelium-dependent vasodilation in patients with insulin-dependent diabetic mellitus. Circulation 1993;88:2510–2516.
McVeigh GE, Brennan GM, Johnston GD, et al. Impaired endothelium-dependent and independent vasodilation in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 1992;35:771–776.
Pieper GM, Jordan M, Dondlinger LA, Adams MB, Roza AM. Peroxidative stress in diabetic blood vessels: Reversal by pancreatic islet transplantation. Diabetes 1995;44:884–889.
Pieper GM, Siebeneich W, Roza AM, Jordan M, Adams MB. Chronic treatment in vivo with dimethylthiourea, a hydroxyl radical scavenger, prevents diabetes-induced endothelial dysfunction. J Cardiovasc Pharmacol 1996;28:741–745.
Pieper GM, Gross GJ. Selective impairment of endothelium-dependent relaxation by oxygen-derived free radicals: Distinction between receptor versus nonreceptor mediators. Blood Vessels 1989;26:44–47.
Pieper GM, Gross GJ. Priming by platelet-activating factor of neutrophil-induced impairment of endothelium-dependent relaxation. J Vasc Med Biol 1990;2:56–61.
Simon BC, Haudenschild CC, Cohen RA. Preservation of endothelium-dependent relaxation in atherosclerotic rabbit aorta by probucol. J Cardiovasc Pharmacol 1993;21:893–901.
Tesfamariam B, Cohen RA. Free radicals mediate endothelial cell dysfunction caused by elevated glucose. Am J Physiol 1992;263 (Heart Circ Physiol 32):H321-H326.
Braughler JM, Pregenzer JF, Chase RL, Duncan LA, Jacobsen EJ, McCall JM. Novel 21-aminosteroids as potent inhibitors of iron-dependent lipid peroxidation. J Biol Chem 1987;262:10438–10440.
Hall ED, Yonkers PA, McCall JM, Braughler JM. Effects of 21-aminosteroid U74006F on experimental head injury in mice. J Neurosurg 1988;68:456–461.
Killinger WA Jr, Dorofi DB, Keagy BA, Johnson G Jr. Improvement of endothelial cell viability at 4°C by addition of lazaroid U74500A to preservation solutions. Transplantation 1992;53:983–986.
Niederau C, Schulz H-U, Klonowski H. Lazaroids protect isolated rat pancreatic acinar cells against damage induced by free radicals. Pancreas 1995;11:107–121.
Aust SD. Lipid peroxidation. In: Greenwald RA, ed. Handbook of Methods for Oxygen Radical Research. Boca Raton, FL: CRC Press, 1985:204–207.
Aebi H. Catalase in vitro. In: Packer L, ed. Methods in Enzymology. Toronto, Canada: Academic Press, 1984:121–126.
Pieper GM, Langenstroer P, Gross GJ. Hydroxyl radicals mediate injury to endothelium-dependent relaxation in diabetic rat. Mol Cell Biochem 1993;122:139–145.
Van Ye TM, Roza AM, Pieper GM, Henderson J Jr, Johnson CP, Adams MB. Inhibition of intestinal lipid peroxidation does not mimic morphologic damage. J Surg Res 1993;55:553–558.
Ovize M, De Lorgeril M, Ovize A, Ciavatti M, Delaye J, Renaud S. U74006F, a novel 21-aminosteroid, inhibits in vivo lipid peroxidation but fails to limit infarct size in a canine model of myocardial ischemia reperfusion. Am Heart J 1991;122:681–689.
Curtis WE, Muldrow ME, Parker NB, Barkley R, Linas SL, Repine JE. N,N′-dimethylthiourea dioxide formation from N,N′-dimethylthiourea reflects hydrogen peroxide concentrations in simple biological systems. Proc Natl Acad Sci USA 1988;85:3422–3425.
Pieper GM, Jordan M, Adams MB, Roza AM. Syngeneic pancreatic islet transplantation reverses endothelial dysfunction in experimental diabetes. Diabetes 1995;44:1106–1113.
Pieper GM, Peltier BA. Amelioration by L-arginine of a dysfunctional arginine/nitric oxide pathway in diabetic endothelium. J Cardiovasc Pharmacol 1995;25:397–403.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pieper, G.M., Jordan, M. & Roza, A.M. Chronic Treatment with the 21-Aminosteroid U74389F, an Inhibitor of Lipid Peroxidation, Does Not Prevent Diabetic Endothelial Dysfunction. Cardiovasc Drugs Ther 11, 435–440 (1997). https://doi.org/10.1023/A:1007785020871
Issue Date:
DOI: https://doi.org/10.1023/A:1007785020871