Abstract
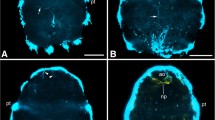
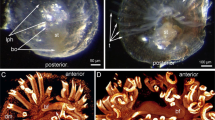
The ultrastructural characteristics of the developing CNS of the pond snail, Lymnaea stagnalis, were investigated, with special attention paid to three specific stages of embryonic development, representing distinctly different phases of both the body morphogenesis and gangliogenesis. These were the 35% (veliger), the 50–55% (metamorphic), and the 75% (post-metamorphic, adult-like) stages of embryonic development. Also, a brief comparison was done with the CNS of hatchlings (100% of embryonic development). During embryogenesis specialized axo-axonic synapses and elements of the glial system, including the ganglionic (neural) sheath, were rarely observed, whereas the frequent occurrence of unspecialized axo-somatic contacts could be demonstrated. Synapse-like axo-axonic connections could be found first in 75% embryos, showing asymmetric vesicle clustering on the “presynaptic” side and increased electron density of the apposed membranes. These phenomena may reflect the dominance of modulatory processes in the CNS during embryogenesis, and the absence of the neural sheath may facilitate trophic and/or hormonal influences within the developing ganglia. The gradual increase in the size of ganglia and the diameter of their neuropils was not accompanied by any widening of the cell body layer or increasing diameter of the nerve cell bodies until the very end of embryogenesis. With respect to the ultrastructural organization of the neuropil, and possibly the entire CNS, a determining stage seems to be that of metamorphosis. Two types of neuropil could be observed at this time; the metamorphosing neuropil with an irregular organization of wavy axon profiles, and well-structured neuropil with a regular organization of axon profiles. Ganglia with irregular or regular neuropil occurred simultaneously in the developing CNS.
Similar content being viewed by others
References
BAILEY, C. H., THOMPSON, E. B., CASTELLUCCI, V. F. & KANDEL, E. R. (1979) Ultrastructure of the synapses of sensory neurons that mediate gill-withdrawal reflex in Aplysia. Journal of Neurocytology 8, 415–44.
BULLOCK, T. H. & HORRIDGE, G. A. (1965) Structure and Function in the Nervous System of Invertebrates. San Francisco: Freeman.
BULLOCH, A. G. M. (1985) Development and plasticity of the molluscan nervous system. In The Mollusca (edited by WILBUR, K. M. & WILLOWS, A. O. D.), pp. 335–408. New York: Academic Press.
CAROLL, D. J. & KEMPF, S. T. (1994) Changes occur in the central nervous system of the nudibranch Berghia verrucornis (Mollusca, Opistobranchia) during metamorphosis. Biological Bulletin 186, 202–212.
CROLL, R. P. & CHIASSON, B. J. (1989) Postembryonic development of serotoninlike immunreactivity in the central nervous system of Lymnaea stagnalis. Journal of Comparative Neurology 280, 122–142.
CROLL, R. P. & VORONEZHSKAYA, E. E. (1996) Early elements in gastropod neurogenesis. Developmental Biology 173, 344–347.
CROLL, R. P., VORONEZHSKAYA, E. E., HIRIPI, L. & ELEKES, K. (1999) Development of catecholaminergic neurons in the pond snail, Lymnaea stagnalis: II. Postembryonic development of central and peripheral cells. Journal of Comparative Neurology 404, 297–309.
ELEKES K. (1978) Ultrastructure of synapses in the central nervous system of lamellibranch molluscs. Acta Biologica Hungarica 29, 139–154.
ELEKES, K. (1991a) Serotonin-immunoreactive varicosities in the cells body region and neural sheath of the snail, Helix pomatia: An electron microscopic immunocytochemical study. Neuroscience 42, 583–591.
ELEKES, K. (1991b) Neurotransmitters in the gastropod CNS: Comparative immunocytochemistry. Acta Biologica Hungarica 43, 213–220.
ELEKES, K. & NÄSSEL, D. R. (1990) Distribution of FMRFamide-like immunoreactive neurons in the central nervous system of the snail Helix pomatia. Cell and Tissue Research 262, 177–190.
ELEKES, K. & UDE, J. (1993) An immunogold electron microscopic analysis of FMRFamide-like immunoreactive neurons in the CNS of Helix pomatia: Ultrastructure and synaptic connections. Journal of Neurocytology 22, 1–13.
ELEKES, K., VEHOVSZKY, Á. & SALÁNKI, J. (1983) Ultrastructure of synaptic connections of a bimodal pacemaker neurons in the central nervous system of Helix pomatia L. Neuroscience 8, 617–629.
ELEKES K., HUSTERT, R. & GEFFARD, M. (1987) Serotonin-immunoreactive and dopamine-immunoreactive neurons in the terminal ganglion of the cricket, Acheta domestica: Light-and electron microscopic immunocytochemistry. Cell and Tissue Research 250, 167–180.
ELEKES, K., VORONEZHSKAYA, E. E., HIRIPI, L., ECKERT, M. & RAPUS, J. (1996) Octopamine in the developing nervous system of the pond snail, Lymnaea stagnalis L. Acta Biologica Hungarica 47, 73–87.
HERNÁDI, L., ELEKES, K. & S.-RÓZSA, K. (1989) Distribution of serotonin-containing neurons in the central nervous system of the snail Helix pomatia. Cell and Tissue Research 257, 313–323.
JACOB, M. H. (1984) Neurogenesis in Aplysia californica resembles nervous system formation in vertebrates. Journal of Neuroscience 4, 1225–1239.
KEMENES, G., ELEKES, K., HIRIPI, L. & BENJAMIN, P. R. (1989) A comparison of four techniques for mapping the distribution of serotonin and serotonin-containing neurons in fixed and living ganglia of the snail, Lymnaea. Journal of Neurocytology 18, 193–208.
KEMPF, S. C., PAGE, L. R. & PIRES, A. (1997) Development of serotonin-like immunoreactivity in the embryos and larvae of nudibranch mollusks with emphasis on the structure and possible function of the apical sensory organ. Journal of Comparative Neurology 386, 507–528.
KRIEGSTEIN, A. R. (1977a) Stages of post-hatching development of Aplysia californica. Journal of Experimental Zooloy 199, 275–288.
KRIEGSTEIN, A. R. (1977b) Development of the nervous system of Aplysia californica. Proceedings of the National Academy of Sciences USA 74, 375–378.
LEITCH, B., LAURENT, G. & SHEPHERD, D. (1992) Embryonic development of synapses on spiking local interneurons in locust. Journal of Comparative Neurology 324, 213–236.
LIN, M.-F. & LEISE, E. M. (1996) Gangliogenesis in the prosobranch Ylianassa obsoleta. Journal of Comparative Neurology 374, 180–193.
LOPRESTI, V., MACAGNO, E. R. & LEVINTHAL, C. (1973) Structure and development of neuronal connections in isogenic organisms: Cellular interaction in the development of the optic lamina of Daphnia. Proceedings of the National Academy of Sciences U.S.A. 70, 433–437.
MACAGNO, E. R. (1979) Cellular interactions and pattern formation in the development of the visual system of Daphnia magna (Crustaces, Branchiopoda). Developmental Biology 15, 1287–1298.
MACAGNO, E. R. (1981) Cellular interactions and pattern formation in the development of the visual system of Daphnia magna (Crustacea, Branchiopoda). II. Induced retardation of optic axon ingrowth results in a delay in laminar neuron differentiation. Journal of Neuroscience 1, 945–955.
MAROIS, R. (1989) Embryonic development of Lymnaea stagnalis: General, neuronal and behavioral aspects.M. Sc. Thesis, Halifax, N. S: Dalhousie University.
MAROIS, R. & CAREW, T. J. (1990) The gastropod nervous system in metamorphosis. Journal of Neurobiology 7, 1053–1071.
MAROIS, R. & CAREW, T. J. (1997a) Fine structure of the apical ganglion and its serotonergic cells in the larva of Aplysia californica. Biological Bulletin 192, 388–398.
MAROIS, R. & CAREW, T. J. (1997b) Ontogeny of serotonergic neurons in Aplysia californica. Journal of Comparative Neurology 386, 477–490.
MAROIS, R. & CAREW, T. J. (1997c) Projection patterns and target tissues of the serotonergic cells in larval Aplysia californica. Journal of Comparative Neurology 386, 491–506.
MAROIS, R. & CROLL, R. P. (1992) Development of serotoninlike immunoreactivity in the embryonic nervous system of the snail Lymnaea stagnalis. Journal of Comparative Neurology 322, 255–265.
MESCHERIAKOV, V. N. (1990) The common pond snail Lymnaea stagnalis L. In Animal Species for Developmental Studies (edited by DETLAFF, D. A. & VASSETZKY, S. G.), pp. 69–132. New York, London: Plenum Press.
MORRILL, J. B. (1982) Development of the pulmonate gastropod, Lymnaea. In Developmental Biology of the Freshwater Invertebrates (edited by HARRISON, F. W. & COWDEN, R. R.), pp. 399–483. New York: Alan P. Liss.
PAGE, L. (1992a) A new interpretation of the nudibranch central nervous system based on ultrastructural analysis of neurodevelopment in Melibe leonina. I. Cerebral and visceral loop ganglia. Biological Bulletin 182, 348–365.
PAGE, L. (1992b) A new interpretation of the nudibranch central nervous system based on ultrastructural analysis of neurodevelopment in Melibe leonina. II. Pedal, pleural and labial ganglia. Biological Bulletin 182, 366–381.
PENTREATH, V. W., RADOJCIC, T., SEAL, L. H. & WINSTANLEY, E. K. (1985) The glial cells and glianeuron relations in the buccal ganglia of Planorbis corneus (L.): Cytological qualitative and quantitative changes during growth and aging. Philosophical Transactions of the Royal Society London (Biology) 307, 399–455.
POGORELAYA, N. K., ELEKES, K. & KISS, I. (1977) Electron microscopic investgation of the a giant neuron identified in the right parietal ganglion of Lymanea stagnalis. Acta Biologica Hungarica 28, 451–460.
RAVEN, C. P. (1966) Morphogenesis: The Analysis of Molluscan Development. New York: Pergamon Press.
REYNOLDS, E. S. (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. Journal of Cell Biology 17, 208–212.
ROUBOS, E. W., BOER, H. H. & SCHOT, L. P. C. (1981) Peptidergic neurons and the control of neuroendocrine activity in the freshwater snail Lymnaea stagnalis (L.). In NeurosecretionÑMolecules, Cells, Systems (edited by FARNER, D. S. & LEDERIS, K.), pp. 119–127. Plenum Press, New York: Plenum Press.
ROUBOS, E. W. & MOORER-VAN DELFT, C. M. (1979) Synaptology of the central nervous system of the freshwater snail Lymnaea stagnalis (L.), with particular reference to neurosecretion. Cell and Tissue Research 198, 217–235.
ROUBOS, E. W. & VAN DER WAL-DIVENDAL, R. M. (1980) Ultrastructural analysis of peptide-hormone release by exocytosis. Cell and Tissue Research 207, 267–275.
SCHACHER, S., KANDEL, E. R. & WOOLLEY, R. (1979a) Development of neurons in the abdominal ganglion of Aplysia californica-I. Axosomatic synaptic contacts. Developmental Biology 71, 163–175.
SCHACHER, S., KANDEL, E. R. & WOOLLEY, R. (1979b) Development of neurons in the abdominal ganglion of Aplysia californica-II. Nonneural support cells. Developmental Biology 71, 176–190.
SELLECK, S. B. & SELLER, H. (1991) The influence of retinal innervation on neurogenesis in the first optic ganglion of Drosophila. Neuron 6, 83–99.
SERFŐZŐ, Z., ELEKES, K. & VARGA, V. (1998) NADPHdiaphorase activity in the nervous system of the embryonic and juvenile pond snail, Lymnaea stagnalis. Cell and Tissue Research 292, 579–586.
SUDLOW, L. C., JIAN, J., MOROZ, L. L. & GILLETTE, R. (1998) Serotonin immunoreactivity in the central nervous system of the marine molluscs Pleuorabranchaea californica and Tritonia diomedea. Journal of Comparative Neurology 395, 466–480.
VAUGHN, J. E. (1989) Review: Fine structure of synaptogenesis in the vertebrate central nervous system. Synapse 3, 255–285.
VORONEZHSKAYA, E. E. & ELEKES, K. (1993) Distribution of serotonin-like immunoreactive neurones in the embryonic nervous system of lymnaeid and planorbid snails. Neurobiology 1, 371–383.
VORONEZHSKAYA, E. E. & ELEKES, K. (1996) Transient and sustained expression of FMRFamide-likeimmunoreactivity in the developing nervous system Lymnaea stagnalis (Mollusca, Pulmonata). Cellular and Molecular Neurobiology 16, 661–676.
VORONEZHSKAYA, E. E., HIRIPI, L., ELEKES, K. & CROLL, R. P. (1999) Development of catecholaminergic neurons in the pond snail, Lymnaea stagnalis: I. Embryonic development of dopamine-containing neurons and dopamine-dependent behaviors. Journal of Comparative Neurology 404, 285–296.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nagy, T., Elekes, K. Embryogenesis of the central nervous system of the pond snail Lymnaea stagnalis L. An ultrastructural study. J Neurocytol 29, 43–60 (2000). https://doi.org/10.1023/A:1007112130414
Issue Date:
DOI: https://doi.org/10.1023/A:1007112130414