Abstract
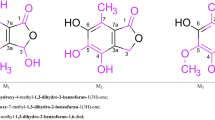
New coordination compounds: [PtIIQ] and [PtIV–CrIIIQ2], where Q = quercetin, were isolated from the [Pt(NH3)2Cl2–quercetin] and [Pt(NH3)2Cl2–CrVI–quercetin] systems, respectively. Structures are proposed on the basis of i.r., n.m.r. and deconvoluted electronic spectra.
Similar content being viewed by others
References
R. Song, K. Mook Kim and Y. Soo Sohn, Inorg. Chim. Acta, 292, 238 (1999).
Z. Guo and P.J. Sadler, Angew. Chem. Int. Ed., 38, 1513 (1999).
Wong and Ch. M. Giandomenico, Chem. Rev., 99, 2451 (1999).
J. Kuduk-Jaworska, Archiv. Immunol. Ther. Exp., 42, 77 (1994).
Proc. 8th Int. Symp. Platinum and other Metal Coordination Compounds in Cancer Chemotherapy, Oxford, 1999.
J. Reedijk, Chem. Rev., 99, 2499 (1999).
B. Lippert, in B. Lippert (Ed) cis-platin, Chemistry and Biochem-istry of a Leading Anticancer Drug, Wiley, Zürich, 1999, p. 230.
B. Lippert, Coordination Chem. Revs., 1, 5 (1999).
E. Wiltshaw and B. Carr, Recent Results in Cancer Res., 48, 178 (1974).
E. Wiltshaw and T. Kroner, Cancer Treat. Rep., 60, 55 (1976).
R.C. Young, D.D. van Hoff, P. Gormley, R. Makuch, J. Cassidy, D. Howser and J.M. Bull, Cancer Treat. Rep., 63, 1539 (1979).
P. Bonomi, J.A. Blessing, F.B. Stehman, P.J. DiSaia, L. Walton and F.J. Major, J. Clin. Oncol., 3, 1079 (1985).
E. Hans, Staying Healthy with Nutrition. Berkeley (CA): Celestial Arts, 1992, p. 307.
J.A. Duke, http://www.ars-grin.gov/duke/. US Dept. of Agri-culture Phytochemical and Ethnobotanical Data Base. 1999, Washington, DC.
J.E. Stansbury, Cancer Prevention Diet, http://www.nutritionscien-cenews.com/index/.
M. Poot, A. Schuster and H. Hoehn, Biochem. Pharmacol., 12, 1903 (1991).
E. Jäger, O. Klein, B. Wächter, H. Bernhard, W. Dippold, K.H. Meyer, Z. BuÈschenfelde and A. Knuth, Oncology, 53, 58 (1996).
G. Scambia, F.O. Raneletti, P. Bennedetti Pancini, M. Piantelli, G. Bonanno, R. De Vincenzo, G. Ferrandina, N. Maggiano, A. Capelli and S. Mancuso, Gyn. Onc., 45, 13 (1992).
W. Deng, X. Fang and J. Wu, Radiat. Phys. Chem., 50, 271 (1997).
C. Alarcon de la Lastra, M.J. Martin and V. Motilva, Pharmacology, 48, 56 (1994).
A.F. Welton, L.D. Tobias and C. Fiedler-Nagy, Prog. Clin. Biol. Res., 213, 231 (1986).
L.M. Larocca, L. Teofili and G. Leone, Br. J. Haematol., 79, 562 (1991).
G. Scambia, F.O. Ranelletti, P. Bennedetti Pancini, G. Bonanno, R. De Vincenzo, M. Piantelli and S. Mancuso, Anti-Cancer Drugs, 1, 45 (1990).
M.K. Kuhlmann, E. Horsch, G. Burkhardt, M. Wagner and H. Kohler, Arch. Toxicol., 72, 536 (1998).
S. Mancuso, G. Folchitto, P.L. Benedetti-Pacini, M. Piantelli, F.O. Ranetelli and A. Cappelli, Patent 92,13851, C.A. 117, 233705h.
G.M. Escandar and L.F. Sala, Can. J. Chem., 69, 1994 (1991).
J. Pusz and M. Kopacz, Pol. J. Chem., 66, 1935 (1992).
J. Stawińska, Z. Staszak, J. Jezierska, M. Cieślak-Golonka and M. Daszkiewicz, Pol. J. Chem., 76, 291 (2000).
G. Bierman and H. Ziegler, Anal. Chem., 58, 536 (1986).
J. Myrczek, Spectr. Lett., 23, 1027 (1990).
C.J. Balhausen, Pure Appl. Chem., 44, 13 (1975).
M.S. Kharasch and T.A. Ashoford, J. Am. Chem. Soc., 58, 1776 (1936).
M. Kodaka, Y. Dohta, P. Rekonen, T. Okada and H. Okuno, Biophysical Chem., 75, 259 (1998).
W.P. Nikitin and M.T. Golovkina, Zh. Obsh. Khim., 53, 1625 (1983).
V. KuntiĈ, S. BlagojeviĈ, D. Malešev, Z. RadoviĈ and M. Bogavac, Monatsh. Chem., 129, 41 (1998).
M.T. Golovkina, S.W. Karavan and M.W. Pokrovskaja, Zh. Obsh. Khim., 44, 2569 (1974).
E. Makasheva and M.T. Golovkina, Zh. Obsh. Khim., 43, 1640 (1973).
E. Balogh-Hergovich, J. Kaizer and G. Speier, Inorg. Chim. Acta, 256, 9 (1997).
L. Nagy, H. Mehner, A.A. Christy, E. Sletten, F.T. Edelman and Q.M. Andersen, J. Radioanal. Nucl. Chem., 227, 89 (1998).
M.E. Bodini, G. Copia, R. Tapia, F. Leighton and L. Herrera, Polyhedron, 18, 2233 (1999).
J.E. Brown, H. Khodr, R.C. Hider and C.A. Rice-Evans, Biochem., 330, 1173 (1998).
1st Conference Flavonoids and their Application, Rzeszów, 1996 (in Polish).
K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, Wiley, New York 1997, 5th edit., Pt B, pp. 7, 169 and 172.
D.J. Liston and B.O. West, Inorg. Chem., 24, 1568 (1985).
A.B.P. Lever, Inorganic Electronic Spectroscopy, Elsevier, 2nd edit., Studies in Physical and Theoretical Chemistry 33, 1984 pp. 344, 466 and 544.
K. Nakamoto, P.J. McCarthy, J. Fujita, R.A. Condrate and G.T. Behnke, Inorg. Chem., 1, 36 (1965).
W.R. Mason and H.B. Gray, J. Am. Chem. Soc., 9, 5721 (1968).
N. Farrel, Y. Qu, U. Bierbach, M. Vaalsecchi and E. Menta, in B. Lippert (Ed), cis-platin, Chemistry and Biochemistry of a Leading Anticancer Drug, Wiley, Zürich, 1999, pp. 479.
B. Lippert and G. Raudaschil, Inorg. Chim. Acta, 78, 161 (1983).
D. Neugebauer and B. Lippert, Inorg. Chim. Acta, 67, 151 (1982).
P. Braunstein, T. Stährfeldt and J. Fischer, C.R. Acadi., Sci. Paris, 2, 273 (1999).
J.M. Casas, J. Fornis and A. Martin, Inorg. Chem., 35, 6009 (1996).
W.Śliwa and M. Deska, Collect. Czech. Chem. Commun., 64, 435 (1999).
B.W. Pfenning, J.V. Lockard, J.L. Cohen, D.F. Watson, D.M. Ho and A.B. Bocarsly, Inorg. Chem., 38, 2941 (1999) and refs therein.
G. Galuszka, M. Cieślak-Golonka and A. Szeląg, Polyhedron, 21, 3785 (1998).
J.M. Burke, J.R. Kincaid and T.G. Spiro, J. Am. Chem. Soc., 19, 6077 (1978).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stawińska, J., Cieślak-Golonka, M., Staszak, Z. et al. The reactivity of cis-platin. Spectroscopic properties of products isolated from the [cis-Pt(NH3)2Cl2–quercetin] and [cis-Pt(NH3)2Cl2–CrVI–quercetin] systems. Transition Metal Chemistry 26, 153–159 (2001). https://doi.org/10.1023/A:1007108104803
Issue Date:
DOI: https://doi.org/10.1023/A:1007108104803