Abstract
1. The potential functions of the microtubule-associated protein tau have been expanded by the recent demonstration of its interaction with the plasma membrane. Since the association of tau with microtubules is regulated by phosphorylation, herein we examine whether or not the association of tau with the plasma membrane is also regulated by phosphorylation.
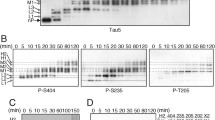
2. A range of tau isoforms migrating from 46 to 64 kDa was associated with crude particulate fractions derived from SH-SY-5Y human neuroblastoma cells, and were retained during the initial stages of plasma membrane purification. During the extensive washing utilized in purification of the plasma membrane, portions of each of these isoforms were depleted from the resultant purified membrane. Immunoblot analysis with phospho-dependent and -independent antibodies revealed selective depletion of phospho isoforms during membrane washing. This effect was more pronounced for the slowest-migrating (64-kDa) tau isoform.
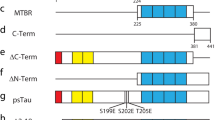
3. This putative influence of phosphorylation on the association of tau with the plasma membrane was further probed by transfection of SH-SY-5Y human neuroblastoma cells with a tau construct that could associate with the plasma membrane but not with microtubules. Treatment with phorbol ester or calcium ionophore, both of which increased phospho-tau levels within the cytosol and plasma membrane, was accompanied by the dissociation of this tau construct from the membrane.
4. These data indicate that phosphorylation regulates the association with the plasma membrane. Dissociation from the membrane by phosphorylation may place tau at risk for hyperphosphorylation and ultimate PHF formation in a manner previously considered for tau dissociated from microtubules.
Similar content being viewed by others
REFERENCES
Aizawa, H., Kawasaki, H., Murofushi, S., Kotani, K., Suzuki, and Sakai, H. (1988). Microtubule-binding domain of tau proteins. J. Biol. Chem. 263:7703-7707.
Alonso, A. D., Grundke-Iqbal, I., Barra, H. S., and Iqbal, K. (1997). Abnormal phosphorylation of tau and the mechanism of Alzheimer neurofibrillary degeneration: Sequestration of microtubuleassociated proteins 1 and 2 and the disassembly of microtubules by the abnormal tau. Proc. Natl. Acad. Sci. USA 94:298-303.
Arias, C., Arrieta, I., and Tapia, R. (1995). ?-Amyloid peptide fragment 25-35 potentiates the calciumdependent release of excitatory amino acids from depolarized hippocampal slices. J. Neurosci. Res. 41:561-566.
Arioka, M., Tsukamoto, M., Ishiguro, K., Kato, R., Sato, K., Imahori, K., and Uchida, T. (1993). Tau protein kinase-1 is involved in the regulation of the normal phosphorylation state of tau protein. J. Neurochem. 60:461-468.
Baas, P. W., Pienkowski, T. P., Cimbalnik, K. A., Toyama, K., Bakalis, S., Ahmand, F. J., and Kosik, K. S. (1994). Tau confers drug-stability but cold-stability to microtubules in living cells. J. Cell Sci. 107:135-143.
Baudier, J., and Cole, R. D. (1987). Phosphorylation of tau proteins to a state like that in Alzheimer's brain in chatalyzed by a calcium/calmodulin-dependent kinase and modulated by phospholipids. J. Biol. Chem. 262:17577-17583.
Binder, L. I., Frankfurter, A., and Rebhun, L. I. (1985). The distribution of tau in the mammalian central nervous system. J. Cell Biol. 101:1371-1378.
Black, M. M., Slaughter, T., Moshiach, M., Obrocka, M., and Fischer, I. (1996). Tau is enriched on dynamics microtubules in the distal region of growing axons. J. Neurosci. 16:3601-3619.
Blanchard, B. J., Raghunandan, R. D., Roeder, H. M., and Ingram, V. M. (1994). Hyperphosphorylation of human tau by brain kinase PK40erk beyond phosphorylation by cAMP-dependent PKA: Relation to Alzheimer's disease. Biochem. Biophys. Res. Commun. 200:187-194.
Boyce, J. J., and Shea, T. B. (1997). Phosphorylation events mediated by protein kinase C? and ? participate in the regulation of tau steady-state levels and generation of certain ''Alzheimer-like'' phospho-epitopes. Int. J. Dev. Neurosci. 15:295-307.
Brambett, G. T., Goedert, M., Jakes, R., Merrick, S. E., Trojanowski, J. Q., and Lee, V. M.-Y. (1993). Abnormal tau phosphorylation at ser-396 in Alzheimer's disease recapitulates development and contributes to reduced microtubule binding. Neuron 10:1089-1099.
Brandt, R., Leger, J., and Lee, G. (1995). Interaction of tau with the neural plasma membrane mediated by tau's amino-terminal projection domain. J. Cell Biol. 131:1327-1340.
Brion, J. P., Guilleminot, J., Couchie, D., Flament, D. J., and Nunez, J. (1988). Both adult and juvenile tau microtubule-associated proteins are axon specific in the developing and adult rat cerebellum. Neuroscience 25:139-146.
Caceres, A., and Kosik, K. S. (1990). Inhibition of neurite polarity by tau antisense oligonucleotides in primary cerebellar neurons. Nature 343:461-463.
Caceres, A., Potrebic, S., and Kosik, K. S. (1991). The effect of tau antisense oligonucleotides on neurite formation of cultured cerebellar macroneurons. J. Neurosci. 11:1515-1523.
Cleveland, D. W., Hwo, S. Y., and Kirschner, M. W. (1977). Purification of tau, a microtubule-associated protein that induces assembly of microtubules from purified tubulin. J. Mol. Biol. 116:227-247.
Cressman, C. M., and Shea, T. B. (1995). Hyperphosphorylation of tau and filopodial retraction following microinjection of protein kinase C catalytic subunits. J. Neurosci. Res. 42:648-656.
Cressman, C. M., and Shea, T. B. (1999). The order of exposure of tau to signal transduction kinases alters the generation of ''AD-like'' phosphoepitopes. Cell. Mol. Neurobiol. 19:223-233.
Cressman, C. M., Mercken, M. M., and Shea, T. B. (1995). Alteration in tau antigenicity and electrophoretic migration by PKC? under cell-free conditions. Neurosci. Res. Commun. 17:61-64.
Dreschel, D. N., Hyman, A. A., Cobb, M. H., and Kirschner, M. W. (1992). Modulation of the dynamic instability of tubulin assembly by the microtubule-associated protein tau. Mol. Biol. Cell 3:1141-1154.
Drubin, D. G., and Kirschner, M. W. (1986). Tau protein function in living cells. J. Cell Biol. 103:2739-2746.
Ekinci, F. J., and Shea, T. B. (1999). Hyperphosphorylation of mitogen-activated protein kinase increases phospho-tau immunoreactivity within human neuroblastoma: Additive and synergistic influence of alteration of additional kinase activities. Cell. Mol. Neurobiol. 19:249-260.
Goedert, M., Jakes, R., Crowther, R. A., Six, J., Lubke, U., Vandermeeren, M., Cras, P., Trojanowski, J. Q., and Lee, V. M.-Y. (1993). The abnormal phosphorylation of tau proteins at ser-202 in Alzheimer's disease recapitulates phosphorylation during development. Proc. Natl. Acad. Sci. USA 90:5066-5070.
Goedert, M., Jakes, R., Crowther, R. A., Cohen, P., Vanmechelein, E., Vandermeeren, M., and Cras, P. (1994). Epitope mapped protein tau are dephosphorylated by protein phosphatase 2A-1. FEBS Lett. 312:195-199.
Goedert, M., Spillantini, M. G., Jakes, R., Crowther, R. A., Vandermeeren, M., Probst, A., Gotz, J., Burki, K., and Cohen, P. (1995). Molecular dissection of the paired helical filament. Neurobiol. Aging 16:325-334.
Gray, C. W., and Patel, A. J. (1995). Neurodegeneration mediated by glutamate and ?-amyloid peptide: A comparison and possible interaction. Brain Res. 691:169-179.
Grundke-Iqbal, I., Iqbal, K., Quinlan, M., Tung, Y. C., Zaidi, M. S., and Wisniewski, H. M. (1986). Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J. Biol. Chem. 261:6084-6089.
Hagestedt, T., Lichtenberg, B., Wille, H., Mandelkow, E.-M., and Mandelkow, E. (1989). Tau protein becomes long and stiff upon phosphorylation: Correlation between paracrystalline structure and degree of phosphorylation. J. Cell Biol. 109:1643-1651.
Hartmann, H., Eckert, A., and Muller, W. E. (1993). ?-Amyloid protein amplifies calcium signalling in central neurons from the adult mouse. Biochem. Biophys. Res. Commun. 194:1216-1220.
Himmler, A., Dreschel, D., Kirschner, M. W., and Martin, D. W. (1989). Tau consists of a set of proteins with repeated C-terminal microtubule-binding domains and variable N-terminal domains. Mol. Cell. Biol. 9:1381-1388.
Iqbal, K., Grundke-Iqbal, I., Zaidi, T., Merz, P. A., Wen, G. Y., Shaikh, S. S., Wisniewski, H. M., Alafuzoff, I., and Winblad, B. (1986). Defective brain microtubule assembly in Alzheimer's disease. Lancet 2:421-426.
Johnson, G. V. W., and Foley, V. G. (1993). Calpain-mediated proteolysis of microtubule-associated protein 2 (MAP2) is inhibited by phosphorylation by cAMP-dependent protein kinase, but not by Ca2+/calmodulin-dependent protein kinase II. J. Neurosci. Res. 34:642-647.
Johnson, G. V. W., Jope, R. S., and Binder, L. I. (1989). Proteolysis of tau by calpain. Biochem. Biophys. Res. Commun. 163:1505-1511.
Kempf, M., Clement, A., Faissner, A., Lee, G., and Brandt, R. (1996). Tau binds to the distal axon early in development of polarity in a microtubule-and microfilament-dependent manner. J. Neurosci. 16:5583-5592.
Kosik, K. S. (1997). Tau: Structure and function. In Avila, J., Brandt, R., and Kosik, K. S. (eds.), Brain Microtubule Associated Proteins, Harwood Academic, Amsterdam, pp. 43-52.
Leger, J. G., Brandt, R., and Lee, G. (1994). Identification of tau protein regions required for process formation in PC12 cells. J. Cell Sci. 340:3-12.
Lee, G., Neve, R. L., and Kosik, K. S. (1989). The microtubule binding domain of tau protein. Neuron 2:1615-1624.
Lee, G., Newman, S. T., Gard, D. L., Band, H., and Panchamoorthy, G. (1998). Tau interacts with src-family non-receptor tyrosine kinases. J. Cell Sci. 111:3167-3177.
Litersky, J. M., Scott, C. W., and Johnson, G. V. W. (1993). Phosphorylation, calpain proteolysis and tubulin binding of recombinant tau isoforms. Brain Res. 604:32-40.
Mandall, J. W., and Banker, G. A. (1996). Microtubule-associated proteins, phosphorylation gradients and the establishment of neuronal polarity. Perspect. Dev. Neurobiol. 4:125-135.
Mandelkow, E.-M., and Mandelkow, E. (1998). Tau in Alzheimer's disease. Trends Cell Biol. 8:425-427.
Mattson, M. P., Cheng, B., Davis, D., Bryant, K., Lieberburg, I., and Rydel, R. E. (1992). ?-Amyloid peptides destabilized calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J. Neurosci. 12:376-389.
Mattson, M. P., Tomaselli, K. J., and Rydel, R. E. (1993). Calcium-destabilizing and neurodegenerative effects of aggregated ?-amyloid peptide are attenuated by basic FGF. Brain Res. 621:35-49.
Raghunandan, R., and Ingram, V. M. (1995). Hyperphosphorylation of the cytoskeletal protein tau by the MAP-kinase PK40erk: Regulation by prior phosphorylation with cAMP-dependent protein kinase A. Biochem. Biophys. Res. Commun. 215:1056-1066.
Sengupta, A., Wu, Q., Grunde-Iqbal, I., Iqbal, K., and Singh, T. J. (1997). Potentiation of GSK-3-catalyzed Alzheimer-like phosphorylation of human tau cdk5. Mol. Cell. Biochem. 167:99-105.
Shea, T. B. (1996). Induction of lysosomal abnormalities and tau hyperphosphorylation in human neuroblastoma cells by colchicine and okadaic acid: Evidence that microtubule disruption contributes to Alzheimer neurodegeneration. Neurosci. Res. Commun. 19:27-36.
Shea, T. B. (1997). Phospholipids alter tau conformation, phosphorylation, proteolysis, and association with microtubules: Implication for tau function under normal and degenerative conditions. J. Neurosci. Res. 50:114-122.
Shea, T. B., and Beermann, M. L. (1994). Respective roles of neurofilaments, microtubules, MAP1B and tau in the outgrowth and stabilization of axonal neurites. Mol. Biol. Cell. 5:863-875.
Shea, T. B., Beermann, M. L., Nixon, R. A., and Fischer, I. (1992). Microtubule-associated protein tau is required for axonal elaboration by neuroblastoma cells. J. Neurosci. Res. 32:363-374.
Shea, T. B., Spencer, M. I., Beermann, M. L., Cressman, C. M., and Nixon, R. A. (1996). Calcium influx into human neuroblastoma cells induces ALZ-50 immunoreactivity: Involvement of calpainmediated hydrolysis of protein kinase C. J. Neurochem. 66:1539-1549.
Shea, T. B., Parabhakar, S., and Ekinci, F. J. (1998). ?-Amyloid and ionophore-mediated calcium influx evoke neurodegeneration by distinct intracellular pathways: Differential involvement of the calpain/ protein kinase C system. J. Neurosci. Res. 49:759-768.
Singh, T. J., Zaidi, T., Grunde-Iqbal, I., and Iqbal, K. (1994a). Modulation of GSK-3 catalyzed phosphorylation of microtubule-associated protein tau by non-proline dependent protein kinases. FEBS Lett. 358:4-8.
Singh, T. J., Haque, N., Grunde-Iqbal, I., and Iqbal, K. (1994b). Rapid Alzheimer-like phosphorylation of tau by the synergistic actions of non-proline dependent kinase and GSK-3. FEBS Lett. 358:267-272.
Steiner, B., Mandelkow, E.-M., Biernat, J., Gustke, N., Meyer, H. E., Schmidt, B., Mieskes, G., Soling, H. D., Dreschel, D., Kirschner, M. W., Goedert, M., and Mandelkow, E. (1990). Phosphorylation of microtubule-associated protein tau: Identification of the site for Ca2+-calmodulin dependent kinase and relationship with tau phosphorylation in Alzheimer tangles. EMBO J. 9:3539-3544.
Takemura, R., Okabe, S., Umeyama, T., Kanai, Y., Cowan, N. I., and Hirokawa, N. (1992). Increased microtubule stability and alpha-tubulin acetylation in cells transfected with microtubule-associated proteins MAP1B, MAP2 or tau. J. Cell Sci. 103:953-964.
Trojanowski, J. Q., Schuck, T., Schmidt, M. L., and Lee, V. M.-Y. (1989). Distribution of tau proteins in the normal human central and peripheral nervous system. J. Histochem. Cytochem. 37:209-215.
Ueda, K., Shinohara, S., Yagami, T., Asakura, K., and Kawasaki, K. (1997). Amyloid beta protein potentiates Ca2+ influx through L-type voltage-sensitive Ca2+ channels: A possible involvement of free radicals. J. Neurochem. 68:265-271.
Yang, L.-S., and Ksiezak-Reding, H. (1995). Calpain-induced proteolysis of normal human tau and tau associated with paired helical filaments. Eur. J. Biochem. 233:9-17.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ekinci, F.J., Shea, T.B. Phosphorylation of Tau Alters Its Association with the Plasma Membrane. Cell Mol Neurobiol 20, 497–508 (2000). https://doi.org/10.1023/A:1007075115574
Issue Date:
DOI: https://doi.org/10.1023/A:1007075115574