Abstract
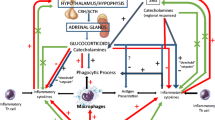
Exercise modulates the macrophage activity via ‘stress hormones’. Three experiments were performed. (1) The effect of strenuous exercise performed by trained mice on macrophage chemotactic capacity was evaluated; (2) peritoneal macrophages from control mice were incubated with plasma from exercised mice or control mice and the differences in chemotaxis were measured; (3) changes in plasma T3 and T4 levels after exercise were measured, and the effect of incubation with the post-exercise levels of plasma T3 and T4 on chemotaxis was then studied in vitro. A 104-fold higher concentration of each hormone was also evaluated. Exercise provoked an increase in chemotaxis (104 ± 35 vs. 47 ± 11 in controls). Incubation with plasma from exercised mice led to an increased level of chemotaxis. Incubation with concentrations of T3 and T4 similar to those observed in post-exercise plasma (T3, 2.3 nmol l-1; T4, 84 nmol l-1) enhanced chemotaxis with respect to incubation with the basal concentrations of the hormones in control animals. A 10M4-fold concentration of T4 reversed this effect. It is concluded that thyroid hormones stimulate macrophage chemotaxis. Also, these data support the hypotheseis that thyroid hormones may be involved in exercise-induced stimulation of chemotaxis.
Similar content being viewed by others
References
Pedersen BK: Immune responses to acute exercise. In: L Hoffman-Goetz (ed). Exercise and Immune Function, CRC Press, Boca Raton, 1996, pp 79–92
Shephard RJ, Shek PN: Exercise training and immune function. In: L Hoffman-Goetz (ed). Exercise and Immune Function, CRC Press, Boca Raton, 1996, pp 93–119
Fitzgerald L: Exercise and the immune system. Immunol Today 11: 337–339, 1988
Fehr HG, Lötzerich H, Michna H: The influence of physical exercise on peritoneal macrophage functions: Histochemical and phagocytic studies. Int J Sports Med 9: 77–81, 1988
Fehr HG, Lötzerich H, Michna H: Human macrophages function and physical exercise: Phagocytic and histochemical studies. Eur J Appl Physiol 58: 613–617, 1989
Ortega E: Influence of exercise on phagocytosis. Int J Sports Med 15: S172–178, 1994
Hoffman-Goetz L, Pedersen BK: Exercise and the immune system: A model of stress response? Immunol Today 15: 382–387, 1994
Simon HB: Exercise and human immune function. In: R Ader, DL Felten, N Cohen (eds). Psychoneuroimmunology, Academic Press, 1991, pp 869–895
Doherty DE, Haslett C, Tonnesen MG, Henson PM: Human monocytes adherence: A primary effect of chemotactic factors on the monocyte to stimulate adherence to human endothelium. J Immunol 138: 1762–1771, 1987
Forner MA, Barriga C, Ortega E: Exercise-induced stimulation of murine macrophage phagocytosis may be mediated by thyroxine. J Appl Physiol 80: 899–903, 1996
Forner MA, Barriga C, Rodríguez AB, Ortega E: A study of the role of corticosterone as a mediator in exercise-induced stimulation of murine macrophage phagocytosis. J Physiol 488.3: 789–794, 1995
Ortega E, Rodríguez MJ, Barriga C, Forner MA: Corticosterone, prolactin and thyroid hormones as hormonal mediators of the stimulated phagocytic capacity of peritoneal macrophages after highintensity exercise. Int J Sports Med 17: 149–155, 1996
Ortega E, Forner MA, Barriga C: Exercise-induced stimulation of murine macrophage chemotaxis: Role of corticosterone and prolactin as mediators. J Physiol 498: 729–734, 1997
Ferry A, Weil B, Amiridis I, Laziry F, Rien M: Splenic immunomodulation with swimming-induced stress in rats. Immunol Lett 29: 261–264, 1991
De la Fuente M: Changes in the macrophage function with aging. Comp Biochem Physiol 81: 935–938, 1985
Ortega E, Forner MA, Barriga C: Effect of β-endorphin on adherence, chemotaxis and phagocytosis of Candida albicans by peritoneal macrophages. Comp Immunol Microbiol Infect Dis 19: 267–274, 1996
Boyden S: The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leukocytes. J Exp Med 114: 453–456, 1962
Forner MA, Collazos ME, Barriga C, De la Fuente M, Rodríguez AB, Ortega E: Effect of age on adherence and chemotaxis capacities of peritoneal macrophages. Influence of physical activity stress. Mech Ageing Dev 75: 179–189, 19940
Ortega E, Collazos ME, Barriga C, De la Fuente M: Stimulation of the phagocytic function in guinea pig peritoneal macrophages by physical activity stress. Eur J Appl Physiol 64: 323–327, 1992
Ortega E, Barriga C, De la Fuente M: Study of the phagocytic process 47 in neutrophils from elite sportswomen. Int J Sports Med 66: 37–42, 1993
Eskola J, Ruuskanen O, Soppi E, Viljanen MK, Jarvinen M, Toivonen P, Kovalainen K: Effect of sport stress on lymphocyte transformation and antibody formation. Clin Exp Immunol 3: 339–345, 1978
Nieman DC, Miller AR, Henson DA, Warren BJ, Gusewitch G: Effect of high-vs. moderate-intensity exercise on natural killer cell activity. Med Sci Sports Exercise 25: 1126–1134, 1993
Monjan AA, Collector MI: Stress-induced modulation of the immune response. Science 196: 307–308, 1977
Dohms JE, Metz A: Stress. Mechanism of immunosuppression. Vet Immunol Immunopathol 30: 89–109, 1991
Luo M, Faure R, Tong YA, Dussault JM: Immunocytochemical localization of the nuclear 3,5,3´-triiodothyronine receptor in the adult rat: Liver, kidney, heart, lung and spleen. Acta Endocrinol Copenhag 120: 451–458, 1989
Costa-Rosa LF, Cury Y, Cury R: Hormonal control of macrophage function and glutamine metabolism. Biochem Cell Biol 69: 309–312, 1991
Khansari DN, Murgo AJ, Faith RE: Effects of stress on the immune system. Immunol Today 11: 170–175, 1990
Fabris N: Immunodepression in thyroid-deprived animals. Clin Exp Immunol 15: 601–611, 1973
Fabris N, Mocchegiane E: Endocrine control of thymic serum factor production in young-adult and old mice. Cell Immunol 91: 325–335, 1985
Fabris N, Muzzioli M, Mocchegiani E: Recovery of age-dependent immunological deterioration in BALB/c mice by short-term treatment with L-thyroxine. Mech Ageing Dev 28: 327–338, 1982
Aoki N, Wakisska G, Nagata I: Effects of thyroxine on T-cell counts and tumor cell rejection in mice. Acta Endocrinol 81: 104–109, 1976
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ortego, E., Forner, M.A., Garcia, J.J. et al. Enhanced chemotaxis of macrophages by strenuous exercise in trained mice: Thyroid hormones as possible mediators. Mol Cell Biochem 201, 41–47 (1999). https://doi.org/10.1023/A:1007020804138
Issue Date:
DOI: https://doi.org/10.1023/A:1007020804138