Abstract
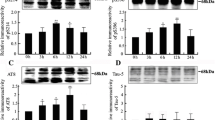
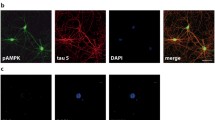
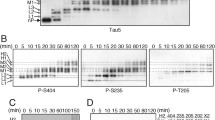
Mitogen-activated protein (MAP) kinase phosphorylates tau in cell-free analyses, but whether or not it does so within intact cells remains controversial. In the present study, microinjection of MAP kinase into SH-SY-5Y human neuroblastoma cells increased tau immunoreactivity toward the phosphodependent antibodies PHF-1 and AT-8. In contrast, treatment with a specific inhibitor of MAP kinase (PD98059) did not diminish “basal” levels of these immunoreactivities in otherwise untreated cells. These findings indicate that hyperactivation of MAP kinase increases phospho-tau levels within cells, despite that MAP kinase apparently does not substantially influence intracellular tau phosphorylation under normal conditions. These findings underscore that results obtained following inhibition of kinase activities do not necessarily provide an indication of the consequences accompanying hyperactivation of that same kinase. Several studies conducted in cell-free systems indicate that exposure of tau to multiple kinases can have synergistic effects on the nature and extent of tau phosphorylation. We therefore examined whether or not such effects could be demonstrated within these cells. Site-specific phospho-tau immunoreactivity was increased in additive and synergistic manners by treatment of injected cells with TPA (which activates PKC), calcium ionophore (which activates calcium-dependent kinases), and wortmannin (which inhibits PIP3 kinase). Alteration in total tau levels was insufficient to account for the full extent of the increase in phospho-tau immunoreactivity. These additional results indicate that multiple kinase activities modulate the influence of MAP kinase on tau within intact cells.
Similar content being viewed by others
REFERENCES
Arioka, M., Tsukamoto, M., Ishiguro, K., Kato, R., Sato, K., Imahori, K., and Uchida, T. (1993). Tau protein kinase-1 is involved in the regulation of the normal phosphorylation state of tau protein. J. Neurochem. 60:461–468.
Bancher, C., Brunner, C., Lassmann, H., Budka, H., Jellinger, K., Wiche, G., Seiteberger, F., Grundke-Iqbal, I., Iqbal, I., and Wisniewski, H. M. (1989). Accumulation of abnormally phosphorylated tau precedes the formation of neurofibrillary tangles in Alzheimer's disease. Brain Res. 477:90–99.
Baudier, J., and Cole, R. D. (1987). Phosphorylation of tau proteins to a state like that in Alzheimer's brain is catalyzed by a calcium/calmodulin-dependent kinase and modulated by phospholipids. J. Biol. Chem. 262:17577–17583.
Blanchard, B. J., Raghunandan, R. D., Roder, H. M., and Ingram, V. M. (1994). Hyperphosphorylation of human tau by brain kinase PK40erk beyond phosphorylation by cAMP-dependent PKA: Relation to Alzheimer's disease. Biochem. Biophys. Res. Commun. 200:187–194.
Boyce, J. J., and Shea, T. B. (1997). Phosphorylation events mediated by protein kinase Cα and ε participate in the regulation of tau steady-state levels and generation of certain “Alzheimer-like” phospho-epitopes. Int. J. Dev. Neurosci. 15:295–307.
Clark, E. A., Leach, K. L., Trojanowski, J. Q., and Lee, V. M. (1991). Characterization and differential distribution of the three major human protein kinase C isozymes (PKC alpha, PKC beta, and PKC gamma) of the central nervous system in normal and Alzheimer's disease brains. Lab. Invest. 64:35–44.
Cressman, C. M., and Shea, T. B. (1995). Hyperphosphorylation of tau and filopodial retraction following microinjection of protein kinase C catalytic subunits. J. Neurosci. Res. 42:648–656.
Cressman, C. M., Boyce, J. B., Wheeler, E., Chang, M., and Shea, T. B. (1995). Site-specific synergism between PKC and MAP kinase in tau hyperphosphorylation in intact SH-SY-5Y human neuroblastoma. J. Neurochem. 67(Suppl.):S62.
Ekinci, F. J., and Shea, T. B. (1997). Tau phosphorylation is regulated by divergent and convergent signal transduction pathways. J. Neurochem. 69(Suppl.):S208.
Felsenstein, K. M., Ingalls, K. M., Hunihan, L. W., and Roberts, S. B. (1994). Reversal of the Swedish familial Alzheimer's disease mutant phenotype in cultured cells treated with phorbol 12,13-dibutyrate. Neurosci. Lett. 174:173–176.
Fowler, C. J., Cowburn, R. F., Garlind, A., Winblad, B., and O'Neill, C. (1995). Disturbances in signal transduction mechanisms in Alzheimer's disease. Mol. Cell. Biochem. 149–150:287–292.
Goedert, M., Jakes, R., Crowther, R. A., Six, J., Lubke, U., Vandermeeren, M., Cras, P., Trojanowski, J. Q., and Lee, V. M.-Y. (1993). The abnormal phosphorylation of tau proteins at Ser-202 in Alzheimer disease recapitulates phosphorylation during development. Proc. Natl. Acad. Sci. USA 90:5066–5070.
Goedert, M., Jakes, R., Crowther, R. A., Cohen, P., Vanmechelein, E., Vandermeeren, M., and Cras, P. (1994). Epitope mapped protein tau are dephosphorylated by protein phosphatase 2A-1. FEBS Lett. 312:195–199.
Govoni, S., Bergamaschi, S., Racchi, M., Battaini, F., Binetti, G., Bianchetti, A., and Trabucchi, M. (1993). Cytosol protein kinase C downregulation in fibroblasts from Alzheimer's disease patients. Neurology 43:2581–2586.
Govoni, S., Racchi, M., Bergamaschi, S., Trabucchi, M., Battaini, F., Bianchetti, A., and Binetti, G. (1996). Defective protein kinase C alpha leads to impaired secretion of soluble beta-amyloid precursor protein from Alzheimer's disease fibroblasts. Ann. N.Y. Acad. Sci. 777:332–337.
Hong, M., and Lee, V. M.-Y. (1997). Insulin and insulin-like growth factor-1 regulate tau phosphorylation in cultured human neurons. J. Biol. Chem. 272:19547–19553.
Imahori, K., and Uchida, T. (1997). Physiology and pathology of tau protein kinases in relation to Alzheimer's disease. J. Biochem. (Tokyo) 121:179–188.
Johnson, G. V. W., and Foley, V. G. (1993). Calpain-mediated proteolysis of microtubule-associated protein 2 (MAP2) is inhibited by phosphorylation by cAMP-dependent protein kinase, but not by Ca2+/calmodulin-dependent protein kinase II. J. Neurosci. Res. 34:642–647.
Kobayashi, S., Ishiguro, K., Omori, A., Takamatsu, M., Arioka, M., Imahora, K., and Uchida, T. (1993). A cdc-related kinase PSSALRE/cdk5 is homologous with the 30kDa subunit of tau protein kinase II, a proline-directed kinase associated with microtubules. FEBS Lett. 335:171–175.
Kosik, K. S., and Greenberg, S. M. (1994). Tau protein and Alzheimer's disease. In Terry, R. D., Katzman, R., and Bick, K. L. (eds.), Alzheimer's Disease, Raven Press, New York, pp. 335–344.
Lanius, R. A., Wagey, R., Sahl, B., Beattie, B. L., Feldman, H., Pelech, S. L., and Krieger, C. (1997). Protein kinase C activity and protein levels in Alzheimer's disease. Brain Res. 764:75–80.
Latimer, D. A., Gallo, J.-M., Lovestone, S., Miller, C. C. J., Reynolds, C. H., Marquardt, B., Stable, S., Woodgett, J. R., and Anderton, B. H. (1995). Stimulation of MAP kinase by v-raf transformation of fibroblasts fails to induce hyperphosphorylation of transfected tau. FEBS Lett. 365:42–46.
Ledesma, M. D., Correase, I., Avila, J., and Diaz-Nido, J. (1992). Implication of brain cdc2 and MAP2 kinases in the phosphorylation of tau protein in Alzheimer's disease. FEBS Lett. 308:218–224.
Lovestone, S., Reynolds, C. H., Latimer, D., Davis, D. R., Anderton, B. H., Gallo, J.-M., Hanger, D., Mulot, S., Marquardt, B., Stabel, S., Woodgett, J. R., and Miller, C. C. J. (1994). Alzheimer's disease-like phosphorylation of the microtubule-associated protein tau by glycogen synthase kinase-3 in transfected mammalian cells. Curr. Biol. 4:1077–1086.
Lu, Q., Soria, J. P., and Wood, J. G. (1993). p44mpk MAP kinase induces Alzheimer type alterations in tau function and in primary hippocampal neurons. J. Neurosci Res. 35:439–444.
Mandelkow, E.-M., Biernat, J., Drewes, G., Gustke, N., Trinczek, B., and Mandelkow, E. (1995). Tau domains, phosphorylation and interactions with microtubules. Neurobiol. Aging 16:355–362.
Masliah, E., Cole, G. M., Hansen, L. A., Mallory, M., Albright, T., Terry, R. D., and Saitoh, T. (1991). Protein kinase C alteration is an early biochemical marker in Alzheimer's disease. J. Neurosci. 11:2759–2767.
Matsushima, H., Shimohama, S., Chachin, M., Taniguchi, T., and Kimura, J. (1996). Ca2+-dependent and Ca2+-independent protein kinase C changes in the brain of patients with Alzheimer's disease. J. Neurochem. 67:317–323.
Mattson, M. P. (1991). Evidence for the involvement of protein kinase C in neurodegenerative changes in cultured human cortical neurons. Exp. Neurol. 112:95–103.
Mattson, M. P., Guthrie, P. B., and Kater, S. B. (1988). Intracellular messengers in the generation and degeneration of hippocampal architecture. J. Neurosci. Res. 20:447–464.
Morishima-Kawashima, M., Hasegawa, M., Takio, K., Suzuki, M., Yoshida, H., Titanti, K., and Ihara, Y. (1995). Proline-directed and non-proline-directed phosphorylation of PHF-tau. J. Biol. Chem. 270:823–829.
Munoz-Montano, J. R., Moreno, F. J., Avila, J., and Diaz-Nido, J. (1995). Lithium inhibits Alzheimer's disease-like tau protein phosphorylation in neurons. FEBS Lett. 411:183–188.
Parrizas, M., Saltiel, A. R., and LeRoith, D. (1997). Insulin-like growth factor 1 inhibits apoptosis using the phosphatidylinositol 3′-kinase and mitogen-activated protein kinase pathways. J. Biol. Chem. 272:154–161.
Pei, J.-J., Sersen, E., Iqbal, K., and Grundke-Iqbal, I. (1994). Expression of protein phosphatases (PP1, PP2A, PP2-B, and PTP-1B) and protein kinases (MAP kinase and p34cdc2) in the hippocampus of patients with Alzheimer disease and normal aged individuals. Brain Res. 655:70–76.
Pope, W. B., Enam, S. A., Bawa, N., Miller, B. E., Ghanbari, H. A., and Klein, W. L. (1993). Phosphorylated tau epitope of Alzheimer's disease is coupled to axon development in the avian central nervous system. Exp. Neurol. 120:106–113.
Pope, W. B., Lambert, M. P., Leypold, B., Seupaul, R., Sletten, L., Krafft, G., and Klein, W. L. (1994). Microtubule-associated protein tau is hyperphosphorylated during mitosis in the human neuroblastoma cell line SH-SY-5Y. Exp. Neurol. 126:185–194.
Pundreddy, S., and Shea, T. B. (1997). AD-like tau phosphorylation in human neuroblastoma cells following PKC hyperactivation is mediated by MAP kinase. Neur. Res. Comm. 21:173–177.
Raghunandan, R., and Ingram, V. M. (1995). Hyperphosphorylation of the cytoskeletal protein tau by the MAP-kinase PK40erk: Regulation by prior phosphorylation with cAMP-dependent protein kinase A. Biochem. Biophys. Res. Commun. 215:1056–1066.
Saitoh, T., Cole, G., and Huynh, T. V. (1990). Aberrant protein kinase C cascades in Alzheimer's disease. Adv. Exp. Med. Biol. 265:301–310.
Sengupta, A., Wu, Q., Grundke-Iqbal, I., Iqbal, K., and Singh, T. J. (1997). Potentiation of GSK-3-catalyzed Alzheimer-like phosphorylation of human tau by cdk5. Mol. Cell. Biochem. 167:99–105.
Shea, T. B. (1997). Phosphatidyl serine alters tau antigenicity, phosphorylation, proteolysis and association with microtubules: Implication for tau function under normal and degenerative conditions. J. Neurosci. Res. 50:114–122.
Shea, T. B., and Cressman, C. M. (1999). The order of exposure of tau to signal transduction kinases alters the generation of “AD-like” phosphoepitopes. Cell. Mol. Neurobiol. 19:223–233.
Shea, T. B., Klinger, E. P., and Cressman, C. M. (1995a). Calcium influx recruits an additional class of kinases to hyperphosphorylate tau. Neuroreport 6:1437–1440.
Shea, T. B., Beermann, M. L., Spencer, M. A., and Nixon, R. A. (1995b). Enhancement of neurite outgrowth following calpain inhibition is mediated by protein kinase C. J. Neurochem. 65:517–527.
Shea, T. B., Spencer, M. J., Beermann, M. L., Cressman, C. M., and Nixon, R. A. (1996). Calcium influx into human neuroblastoma cells induces ALZ-50 immunoreactivity: Involvement of calpainmediated hydrolysis of protein kinase C. J. Neurochem. 66:1539–1549.
Shea, T. B., Parabhakar, S., and Ekinci, F. J. (1997). β-Amyloid and ionophore-mediated calcium influx evoke neurodegeneration by distinct intracellular pathways: Differential involvement of the calpain/protein kinase C system. J. Neurosci. Res. 49:759–768.
Shimohama, S., and Matsushima, H. (1995). Signal transduction mechanisms in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 9:15–22.
Shpetner, H., Joly, M., Hartley, D., and Corvera, S. (1997). Potential sites of PI-3 function in the endocytic pathway revealed by the PI-3 kinase inhibitor, wortmannin. J. Cell Biol. 132:595–605.
Singh, T. J., Zaidi, T., Grundke-Iqbal, I., and Iqbal, K. (1994a). Modulation of GSK-3 catalyzed phosphorylation of microtubule-associated protein tau by non-proline-dependent protein kinases. FEBS Lett. 358:4–8.
Singh, T. J., Haque, N., Grundke-Iqbal, I., and Iqbal, K. (1994b). Rapid Alzheimer-like phosphorylation of tau by the synergistic actions of non-proline dependent kinase and GSK-3. FEBS Lett. 358:267–272.
Steiner, B., Mandelkow, E.-M., Biernat, J., Gustke, N., Meyer, H. E., Schmidt, B., Mieskes, G., Söling, H. D., Dreschsel, D., Kirschner, M. W., Goedert, M., and Mandelkow, E. (1990). Phosphorylation of microtubule-associated protein tau: Identification of the site for Ca2+-calmodulin dependent kinase and relationship with tau phosphorylation in Alzheimer tangles. EMBO J. 9:3539–3544.
Tanaka, T., Iqbal, K., Trenkner, E., Liu, D. J., and Grundke-Iqbal, I. (1995). Abnormally phosphorylated tau in SY5Y human neuroblastoma cells. FEBS Lett. 360:5–9.
Trojanowski, J. Q., Schmidt, M. L., Shin, R.-W., Bramblett, G. T., Rao, D., and Lee, V. M.-Y. (1993a). Altered tau and neurofilament proteins in neurodegenerative diseases: Diagnostic implications for Alzheimer's disease and Lewy body dementias. Brain Pathol. 3:45–54.
Trojanowski, J. Q., Schmidt, M. L., Shin, R.-W., Bramblett, G. T., Goedert, M., and Lee, V. M.-Y. (1993b). PHF tau (A68): from pathological marker to potential mediator of neuronal dysfunction and degeneration in Alzheimer's disease. Clin. Neurosci. 1:184–191.
VanRenterghem, B., Browning, M. D., and Maller, J. L. (1994). Regulation of mitogen-activated protein kinase activation by protein kinases A and C in a cell-free system. J. Biol. Chem. 269:24666–24672.
Vincent, I., Jicha, G., Rosado, M., and Dickson, D. W. (1997). Aberrant expression of mitotic Cdc2/cyclin B1 kinase in degenerating neurons of Alzheimer's disease brain. J. Neurosci. 17:3588–3596.
Wang, H. Y., Pisano, M. R., and Friedman, E. (1994). Attenuated protein kinase C activity and translocation in Alzheimer's disease brain. Neurobiol. Aging 15:293–298.
Wood, J. G., Lu, Q., Reich, C., and Zinsmeister, P. (1993). Proline-directed kinase systems in Alzheimer's disease pathology. Neurosci. Lett. 156:83–86.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ekinci, F.J., Shea, T.B. Hyperactivation of Mitogen-Activated Protein Kinase Increases Phospho-Tau Immunoreactivity Within Human Neuroblastoma: Additive and Synergistic Influence of Alteration of Additional Kinase Activities. Cell Mol Neurobiol 19, 249–260 (1999). https://doi.org/10.1023/A:1006981228331
Issue Date:
DOI: https://doi.org/10.1023/A:1006981228331