Abstract
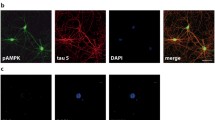
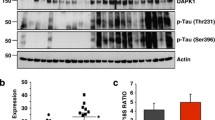
Abnormally hyperphosphorylated tau is the major protein constituent of neurofibrillary tangles (NFTs) in the brain of Alzheimer disease (AD) patients. Cell cycle reactivation is considered an important neuropathological feature of AD, and re-expression and activation of cell cycle regulators are known to occur in neurons containing NFTs. The aim of the present study was to investigate cell cycle reactivation during tau hyperphosphorylation in primary hippocampal neurons. We used forskolin, a specific activator of PKA, to induce tau hyperphosphorylation in cultured primary hippocampal neurons, and then measured levels of cyclin D1 and cyclin B1. We found that forskolin induced hyperphosphorylation of tau at Ser214, Ser396, and Ser202/Thr205 sites, attaining peak levels at 6, 12, and 12 h, respectively, while returning to normal levels at 24 h. Forskolin also induced a sustained cAMP elevation and PKA activation, which peaked at 6 h, in association with activation and overexpression of protein phosphatase-2A (PP-2A) at 24 h. The tau hyperphosphorylation was accompanied by increases in cyclin D1 and cyclin B1 levels; immunostaining showed overlapping distribution of hyperphosphorylated tau and cyclin D1 and cyclin B1 in primary hippocampal neurons. Forskolin induced hyperphosphorylation of tau and increased cyclin D1 and cyclin B1 protein levels in HEK293/tau441 cells, but not in the HEK293/vector cells, whereas the PKA inhibitor H89 inhibited the effects of forskolin on tau hyperphosphorylation and cyclin D1 and cyclin B1 protein levels. These findings suggest that forskolin induces tau hyperphosphorylation, which is itself necessary for the subsequent increases of cyclin D1 and cyclin B1 levels.








Similar content being viewed by others
Change history
07 February 2017
An erratum to this article has been published.
References
Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI (1986) Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A 83(13):4913–4917
Ihara Y, Nukina N, Miura R, Ogawara M (1986) Phosphorylated tau protein is integrated into paired helical filaments in Alzheimer’s disease. J Biochem 99(6):1807–1810
Lee VM, Balin BJ, Otvos L Jr, Trojanowski JQ (1991) A68: a major subunit of paired helical filaments and derivatized forms of normal tau. Science 251(4994):675–678
Iqbal K, Liu F, Gong CX, Grundke-Iqbal I (2010) Tau in Alzheimer disease and related tauopathies. Curr Alzheimer Res 7(8):656–664
Khatoon S, Grundke-Iqbal I, Iqbal K (1994) Levels of normal and abnormally phosphorylated tau in different cellular and regional compartments of Alzheimer disease and control brains. FEBS Lett 351(1):80–84
Kopke E, Tung YC, Shaikh S, Alonso AC, Iqbal K, Grundke-Iqbal I (1993) Microtubule-associated protein tau. Abnormal phosphorylation of a non-paired helical filament pool in Alzheimer disease. J Biol Chem 268(32):24374–24384
Iqbal K, Grundke-Iqbal I, Zaidi T, Merz PA, Wen GY, Shaikh SS, Wisniewski HM, Alafuzoff I et al (1986) Defective brain microtubule assembly in Alzheimer’s disease. Lancet 2(8504):421–426
Braak E, Braak H, Mandelkow EM (1994) A sequence of cytoskeleton changes related to the formation of neurofibrillary tangles and neuropil threads. Acta Neuropathol 87(6):554–567
Iqbal K, Liu F, Gong CX (2016) Tau and neurodegenerative disease: the story so far. Nat Rev Neurol 12(1):15–27. doi:10.1038/nrneurol.2015.225
Martin L, Latypova X, Wilson CM, Magnaudeix A, Perrin ML, Yardin C, Terro F (2013) Tau protein kinases: involvement in Alzheimer’s disease. Ageing Res Rev 12(1):289–309
Tian Q, Zhang JX, Zhang Y, Wu F, Tang Q, Wang C, Shi ZY, Zhang JH et al (2009) Biphasic effects of forskolin on tau phosphorylation and spatial memory in rats. J Alzheimers Dis 17(3):631–642. doi:10.3233/JAD-2009-1088
Liu SJ, Zhang JY, Li HL, Fang ZY, Wang Q, Deng HM, Gong CX, Grundke-Iqbal I et al (2004) Tau becomes a more favorable substrate for GSK-3 when it is prephosphorylated by PKA in rat brain. J Biol Chem 279(48):50078–50088. doi:10.1074/jbc.M406109200
Zhang Y, Li HL, Wang DL, Liu SJ, Wang JZ (2006) A transitory activation of protein kinase-a induces a sustained tau hyperphosphorylation at multiple sites in N2a cells-imply a new mechanism in Alzheimer pathology. J Neural Transm (Vienna) 113(10):1487–1497. doi:10.1007/s00702-005-0421-2
Wang JZ, Wu Q, Smith A, Grundke-Iqbal I, Iqbal K (1998) Tau is phosphorylated by GSK-3 at several sites found in Alzheimer disease and its biological activity markedly inhibited only after it is prephosphorylated by A-kinase. FEBS Lett 436(1):28–34
Martin L, Latypova X, Wilson CM, Magnaudeix A, Perrin ML, Terro F (2013) Tau protein phosphatases in Alzheimer’s disease: the leading role of PP2A. Ageing Res Rev 12(1):39–49. doi:10.1016/j.arr.2012.06.008
Wang JZ, Liu F (2008) Microtubule-associated protein tau in development, degeneration and protection of neurons. Prog Neurobiol 85(2):148–175
Tian Q, Lin ZQ, Wang XC, Chen J, Wang Q, Gong CX, Wang JZ (2004) Injection of okadaic acid into the meynert nucleus basalis of rat brain induces decreased acetylcholine level and spatial memory deficit. Neuroscience 126(2):277–284. doi:10.1016/j.neuroscience.2004.03.037
Wang X, Blanchard J, Tung YC, Grundke-Iqbal I, Iqbal K (2015) Inhibition of protein phosphatase-2A (PP2A) by I1PP2A leads to hyperphosphorylation of tau, neurodegeneration, and cognitive impairment in rats. J Alzheimers Dis 45(2):423–435. doi:10.3233/JAD-142403
Yin YY, Liu H, Cong XB, Liu Z, Wang Q, Wang JZ, Zhu LQ (2010) Acetyl-L-carnitine attenuates okadaic acid induced tau hyperphosphorylation and spatial memory impairment in rats. J Alzheimers Dis 19(2):735–746. doi:10.3233/JAD-2010-1272
Cheng XS, Zhao KP, Jiang X, Du LL, Li XH, Ma ZW, Yao J, Luo Y et al (2013) Nmnat2 attenuates Tau phosphorylation through activation of PP2A. J Alzheimers Dis 36(1):185–195. doi:10.3233/jad-122173
Liu GP, Wei W, Zhou X, Shi HR, Liu XH, Chai GS, Yao XQ, Zhang JY et al (2013) Silencing PP2A inhibitor by lenti-shRNA interference ameliorates neuropathologies and memory deficits in tg2576 mice. Mol Ther 21(12):2247–2257. doi:10.1038/mt.2013.189
Frade JM, Ovejero-Benito MC (2015) Neuronal cell cycle: the neuron itself and its circumstances. Cell Cycle 14(5):712–720. doi:10.1080/15384101.2015.1004937
van Leeuwen LA, Hoozemans JJ (2015) Physiological and pathophysiological functions of cell cycle proteins in post-mitotic neurons: implications for Alzheimer’s disease. Acta Neuropathol 129(4):511–525. doi:10.1007/s00401-015-1382-7
Keeney JT, Swomley AM, Harris JL, Fiorini A, Mitov MI, Perluigi M, Sultana R, Butterfield DA (2012) Cell cycle proteins in brain in mild cognitive impairment: insights into progression to Alzheimer disease. Neurotox Res 22(3):220–230. doi:10.1007/s12640-011-9287-2
Bonda DJ, Lee HP, Kudo W, Zhu X, Smith MA, Lee HG (2010) Pathological implications of cell cycle re-entry in Alzheimer disease. Expert Rev Mol Med 12:e19. doi:10.1017/S146239941000150X
Zhu X, Lee HG, Perry G, Smith MA (2007) Alzheimer disease, the two-hit hypothesis: an update. Biochim Biophys Acta 1772(4):494–502. doi:10.1016/j.bbadis.2006.10.014
Ahn KW, Joo Y, Choi Y, Kim M, Lee SH, Cha SH, Suh YH, Kim HS (2008) Swedish amyloid precursor protein mutation increases cell cycle-related proteins in vitro and in vivo. J Neurosci Res 86(11):2476–2487. doi:10.1002/jnr.21690
Li HL, Wang HH, Liu SJ, Deng YQ, Zhang YJ, Tian Q, Wang XC, Chen XQ et al (2007) Phosphorylation of tau antagonizes apoptosis by stabilizing beta-catenin, a mechanism involved in Alzheimer’s neurodegeneration. Proc Natl Acad Sci U S A 104(9):3591–3596
Rodriguez G, Ross JA, Nagy ZS, Kirken RA (2013) Forskolin-inducible cAMP pathway negatively regulates T-cell proliferation by uncoupling the interleukin-2 receptor complex. J Biol Chem 288(10):7137–7146. doi:10.1074/jbc.M112.408765
Sontag JM, Sontag E (2014) Protein phosphatase 2A dysfunction in Alzheimer’s disease. Front Mol Neurosci 7(16). doi:10.3389/fnmol.2014.00016
Vandamme J, Castermans D, Thevelein JM (2012) Molecular mechanisms of feedback inhibition of protein kinase A on intracellular cAMP accumulation. Cell Signal 24(8):1610–1618. doi:10.1016/j.cellsig.2012.04.001
Itoh Y, Sanosaka M, Fuchino H, Yahara Y, Kumagai A, Takemoto D, Kagawa M, Doi J et al (2015) Salt-inducible kinase 3 signaling is important for the gluconeogenic programs in mouse hepatocytes. J Biol Chem 290(29):17879–17893. doi:10.1074/jbc.M115.640821
Malm HA, Mollet IG, Berggreen C, Orho-Melander M, Esguerra JL, Goransson O, Eliasson L (2016) Transcriptional regulation of the miR-212/miR-132 cluster in insulin-secreting beta-cells by cAMP-regulated transcriptional co-activator 1 and salt-inducible kinases. Mol Cell Endocrinol 424:23–33. doi:10.1016/j.mce.2016.01.010
Sontag JM, Sontag E (2014) Protein phosphatase 2A dysfunction in Alzheimer’s disease. Front Mol Neurosci 7:16. doi:10.3389/fnmol.2014.00016
Ahn JH, McAvoy T, Rakhilin SV, Nishi A, Greengard P, Nairn AC (2007) Protein kinase A activates protein phosphatase 2A by phosphorylation of the B56delta subunit. Proc Natl Acad Sci U S A 104(8):2979–2984. doi:10.1073/pnas.0611532104
Usui H, Inoue R, Tanabe O, Nishito Y, Shimizu M, Hayashi H, Kagamiyama H, Takeda M (1998) Activation of protein phosphatase 2A by cAMP-dependent protein kinase-catalyzed phosphorylation of the 74-kDa B″ (delta) regulatory subunit in vitro and identification of the phosphorylation sites. FEBS Lett 430(3):312–316
Feschenko MS, Stevenson E, Nairn AC, Sweadner KJ (2002) A novel cAMP-stimulated pathway in protein phosphatase 2A activation. J Pharmacol Exp Ther 302(1):111–118
Li M, Wang X, Meintzer MK, Laessig T, Birnbaum MJ, Heidenreich KA (2000) Cyclic AMP promotes neuronal survival by phosphorylation of glycogen synthase kinase 3beta. Mol Cell Biol 20(24):9356–9363
Park CH, Moon Y, Shin CM, Chung JH (2010) Cyclic AMP suppresses matrix metalloproteinase-1 expression through inhibition of MAPK and GSK-3beta. J Invest Dermatol 130(8):2049–2056. doi:10.1038/jid.2010.62
Acknowledgments
This work is supported in part by grants from the National Natural Science Foundation of China (81572222 and 30900725) and Guangdong Provincial Natural Science Foundation (2014A030313333 and 8151051501000005).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
This study using primary cell culture met all institution and national standards for experimental ethics.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
An erratum to this article is available at https://doi.org/10.1007/s12035-017-0415-8.
Rights and permissions
About this article
Cite this article
Wang, HH., Li, Y., Li, A. et al. Forskolin Induces Hyperphosphorylation of Tau Accompanied by Cell Cycle Reactivation in Primary Hippocampal Neurons. Mol Neurobiol 55, 696–706 (2018). https://doi.org/10.1007/s12035-016-0348-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0348-7