Abstract
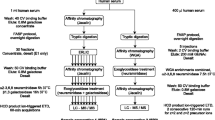
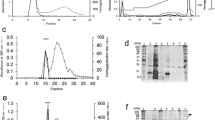
The heat-stable acid-soluble phosphoglycoprotein component PP3 was isolated from the bovine milk proteose peptone fraction by concanavalin A affinity chromatography. Glycopeptides from the ConA-bound fraction corresponding to the component PP3 were obtained by Pronase digestion and were separated by gel filtration into high and low-molecular-mass glycopeptides. In a previous work, we have investigated the structure of the N-glycans from the high-molecular-mass glycopeptides [Girardet et al. (1995) Eur J Biochem 234: 939–46]. Here, we describe the structure of the O-glycans from the low-molecular-mass glycopeptides. By combining methylation analysis, mass spectrometry, 400 MHz 1H-NMR spectroscopy and peptide sequence analysis, we show that the low-molecular-mass fraction contains several neutral glycopeptides. A mixture of the following three glycan structures linked to the Thr86 has been identified: GalNacα1-O-Thr, Gal(β1-3)GalNAcα1-O-Thr and Gal(β1-4)GlcNAc(β1-6)[Galβ1-3)]GalNAcα1-O-Thr. © 1998 Rapid Science Ltd
Similar content being viewed by others
References
Kanno C (1980) J Dairy Sci 72: 883-91.
Girardet JM, Linden G (1996) J Dairy Res 63: 333-50.
Sørensen ES, Petersen TE (1993) J Dairy Res 60: 35-42.
Lasky LA, Singer MS, Dowbenko D, Imai Y. Henzel WJ, Grimley C, Fennie C, Gillet N, Watson SR, Rosen SD (1992) Cell 69: 927-38.
Johnsen LB, Sørensen ES, Petersen TE, Berglund L (1995) Biochim Biophys Acta 1260: 116-8.
Kester JJ, Brunner JR (1982) J Dairy Sci 65: 2241-52.
Nejjar Y, Pâquet D, Godbillon G, Le Deaut JY (1986) Int J Biochem 18: 893-900.
Girardet JM, Coddeville B, Plancke Y, Strecker G, Campagna S, Spik G, Linden G (1995) Eur J Biochem 234: 939-46.
Pâquet D, Nejjar Y, Linden G (1988) J Dairy Sci 71: 1464-71.
Krusius T, Finne J, Ravaula H (1976) FEBS Lett 71: 117-20.
Montreuil J (1980) Adv Carbohydr Chem Biochem 37: 157-223.
Hardy MR, Townsend RR (1988) Proc Natl Acad Sci USA 85: 3289-93.
Zanetta JP, Breckenridge WC, Vincendon G (1972) J Chromatogr 69: 291-304.
Kamerling JP, Gerwig GJ, Vliegenthart JFG, Clamp JR (1975) Biochem J 151: 491-5.
Ciucanu I, Kerek F (1984) Carbohydr Res 131: 209-17.
Fournet B, Strecker G, Leroy Y, Montreuil J (1981) Anal Biochem 116: 489-502.
Vliegenthart JFG, Dorland L, van Halbeek H (1983) Adv Carbohydr Chem Biochem 41: 209-374.
Girardet JM, Saulnier F, Driou A, Linden G, Coddeville B, Spik G (1994) J Dairy Sci 77: 1205-16.
Mäaheimo H, Renkonen R, Turunen JP, Penttila L, Renkonen O (1995) Eur J Biochem 234: 616-25.
Kamerling JP, Vliegenthart JFG (1992) Biological Magnetic Resonance (Berliner LJ, Reben J, eds) vol 10 pp 1-287. New York and London: Plenum Press.
van Halbeek H, Vliegenthart JFG, Fiat AM, Jollès P (1985) FEBS Lett 187: 81-8.
Dua VK, Dube VE, Bush CA (1984) Biochim Biophys Acta 802: 29-40.
Pierce-Crétel A, Decottignies JP, Wieruszeski JM, Strecker G, Montreuil J, Spik G (1989) Eur J Biochem 182: 457-76.
Mollé D, Leonil J (1995) J Chromatogr 708: 223-38.
Pisano A, Packer NH, Redmond JW, Williams KL, Gooley AA (1994) Glycobiology 4: 837-44.
Saito T, Itoh T, Adachi S, Suzuki T, Usui T (1981) Biochim Biophys Acta 678: 257-67.
Sato T, Furukawa K, Greenwalt DE, Kobata A (1993) J Biochem 114: 890-900.
Sato T, Takio K, Kobata A, Greenwalt DE, Furukawa K (1995) J Biochem 117: 147-57.
Nakata N, Furukawa K, Greenwalt DE, Sato K, Kobata A (1993) Biochemistry 32: 4369-83.
Groenen MAM, Dijkhof RJM, van der Poel JJ (1995) Gene 158: 189-95.
Marti T, Schaller J, Rickli EE, Schmid K, Kamerling JP, Gerwig GJ, van Halbeek H, Vliegenthart JFG (1988) Eur J Biochem 173: 57-63.
Dowbenko D, Kikuta A, Fennie C, Gillet N, Laskey LA (1993) J Clin Invest 92: 952-60.
Imai Y, Lasky LA, Rosen SD (1993) Nature 361: 555-7.
Imai Y, Rosen SD (1993) Glycoconjugate J 10: 34-9.
Hemmerich S, Rosen SD (1994) Biochemistry 33: 4830-5.
Hemmerich S, Leffler H, Rosen SD (1995) J Biol Chem 270: 12035-47.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Coddeville, B., Girardet, JM., Plancke, Y. et al. Structure of the O-glycopeptides isolated from bovine milk component PP3. Glycoconj J 15, 371–378 (1998). https://doi.org/10.1023/A:1006973802139
Issue Date:
DOI: https://doi.org/10.1023/A:1006973802139