Abstract
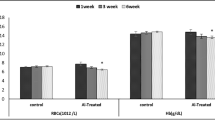
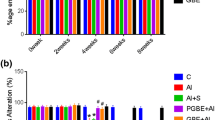
The present study investigates the possible effects of chronic aluminium exposure on the various aspects of calcium homeostasis in the primate central nervous system. Aluminium administration caused a marked decline in the activity of Ca2+ ATPase in the monkey brain. The total calcium content was also significantly raised following aluminium exposure. Concomittant to the increase in the calcium content, the levels of lipid peroxidation were also augmented in the aluminium treated animals, thereby further accentuating the aluminium induced neuronal damage. In addition, aluminium had an inhibitory effect on the depolarization induced 45Ca2+ uptake via the voltage operated channels. The results presented herein, indicate that the toxic effects of aluminium could be mediated through modifications in the intracellular calcium homeostasis with resultant altered neuronal function.
Similar content being viewed by others
References
Kruger GL, Morris TK, Suskind RR, Widner EM: The health effect of aluminium compounds in mammals. Critical Rev Toxicol 13: 1–24, 1984
Salusky IB, Foley J, Nelson P, Goodman WG: Aluminium accumulation during treatment with aluminium hydroxide and dialysis encephalopathy in children and young adults with chronic renal disease. N Engl J Med 324: 527–531, 1991
Candy JM, Oakley AE, Klinowski J: Aluminosilicates and senile plaque formation in Alzheimer's disease. Lancet ii: 354–357, 1986
Perl D P, Gajdusek D C and Garruto R M: Intraneuronal aluminium accumulation in amyotrophic lateral sclerosis and Parkinsonian dementia of Guam. Science 217: 1053–1055, 1982
Perl DP, Brody AR: Alzheimer's disease; X-ray spectrometeric evidence of aluminium accumulation in neurofibrillary tangle bearing neurons. Science 208: 297–299, 1980
Anghileri LJ: Effect of complexed iron and aluminium on brain calcium. Neurotoxicology 13: 475–478, 1992
Gibson GE, Peterson C: Calcium and the aging nervous system. Neurobiol Aging 8: 329–343, 1987
Cheek TR: Calcium regulation and homeostasis. Curr Opinion Cell Biol 3: 199–205, 1–51
Malenka RC, Kauer JC, Perkel DJ, Nicoll RA: The impact of postsynaptic calcium and synaptic transmission-its role in long term potentiation. Trends Neurosci 12: 444–450, 1989
McCormack JG, Halestrap AP, Denton RM: Role of Ca2+ ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev 70: 391–425, 1990
Szekely AM, Costa E, Gayson DR: Transcriptional program coordination by N-methyl-D-aspartate sensitive glutamate receptor stimulation in primary cultures of cerebellar neurons. Mol Pharmacol 38: 624–33, 1990
Lynch G, Baudry M: Biochemistry of memory: A new and specific hypothesis. Science 224: 1057–1063, 1984
Edelfors S, Ravn-Jonsen A: Effect of simultaneous ethanol and toluene exposure on nerve cells measured by changes in synaptosomal Ca2+ uptake and Ca2+ Mg2+ ATPase. Pharmacol Toxicol 69: 90–95, 1991
Desaiah D, Chetty CS, Prasada Rao KS: Chlordecone inhibition of CaM inactivated Ca2+ ATPase in rat brain synaptosomes. J Toxicol Environ Health 16: 189–196, 1985
Fiske CH, Subba Row Y: The colorimetric determination of phosphorus. J Biol Chem 66: 375–400, 1925
Trinder P: Colorometric micro-determination of calcium in serum. Analyst 85: 889–94, 1960
Wilis ED: Mechanism of lipid peroxide formation in animal tissues. Biochem J 99: 667–676, 1966
Lowry O H, Rosebrough N J, Farr A L and Randall R J: Protein measurement with Folin-phenol reagent. J Biol Chem 193: 265–275, 1951
Trapp GA: Interactions of aluminium with cofactors, enzymes and other proteins. Kidney Int 29: S12–S16, 1986
Seigal N, Haug A: Aluminium interaction with calmodulin. Evidence for altered structure and function from optical and enzymatic study. Biochem Biophys Acta 744: 36–45, 1983
Julka D, Gill KD: Altered calcium homeostasis – a possible mechanism of aluminium induced neurotoxicity. Biochem Biophys Acta (in press)
Gibbsons SJ, Brorson JR, Bleakman D, Chard PS, Miller RJ: Calcium influx and neurodegeneration. Ann NY Acad Sci USA 679: 22–33, 1993
Prabhu SD, Salama G: The heavy metal ions Ag2+ and Hg2+ trigger calcium release from cardiac sarcoplasmic reticulum. Arch Biochem Biophys 277: 47–51, 1990
Yamamoto HA: Relations of Ca2+ accumulation and lipid peroxidation with CCl4 induced toxicity in rat liver. Pharmacol Toxicol 66: 213–216, 1990
Choi DW: Calcium mediated neurotoxicity: Relationship to specific channel types and role in ischemic damage. Neuron 1: 623–624, 1988
Mattson MP, Guthrie PB, Kates SB: A role of Na + dependent Ca2+ extrusion in protection against neuronal excitotoxicty. FASEB J 3: 2519–2526, 1989
Gutteridge JMC, Halliwell B: The measurement and mechanisms of lipid peroxidation. Trends Biochem Sci 15: 129–135, 1990
Babizhayev MA: The biphasic effect of calcium on lipid peroxidation. Arch Biochem Biophys 266: 446–451, 1988
Koenig ML, Jope RS: Aluminium inhibits fast phase of voltage-dependent calcium influx into synaptosomes. J Neurochem 49: 316–320, 1987
Nachshen DA: Regulation of cytosolic calcium concentrations in presynaptic nerve endings isolated from rat brain. J Physiol 363: 87–101, 1985
Leslie SW, Chandler LJ, Barr E, Farar RP: Reduced Ca2+ uptake by rat brain mitochondria and synaptosomes in response to aging. Brain Res 329: 177–183, 1985
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sarin, S., Julka, D. & Gill, K.D. Regional alterations in calcium homeostasis in the primate brain following chronic aluminium exposure. Mol Cell Biochem 168, 95–100 (1997). https://doi.org/10.1023/A:1006891125762
Issue Date:
DOI: https://doi.org/10.1023/A:1006891125762