Abstract
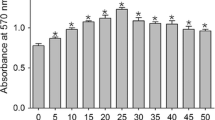
Mineralocorticoids have been implicated in promoting fibrous tissue formation in various organs. In the present study, we sought to address the potential contribution of mineralocorticoids to fibrous tissue formation using a skin pouch model which has proved valuable for the analysis of inflammatory and wound healing responses. Skin pouches were induced in rats by administration of a phorbol ester, croton oil (0.5 ml of a 1% solution). After 2 weeks, rats were killed and intact pouch tissue collected. Pouch weights of control and aldosterone-treated (0.75 μg/h via osmotic minipump) rats were similar (3.33 ± 0.44 g vs. 3.70 ± 0.28 g respectively). However, pouch weights were reduced by more than 50% in spironolactone-treated (25 mg/day powdered in food) animals (1.62 ± 0.22 g and 1.27 ± 0.23 g respectively in aldosterone and spironolactone alone groups). To ascertain the effects of different treatments on collagen accumulation, hydroxyproline concentration was measured. Compared with controls, hydroxyproline concentration was significantly reduced following spironolactone treatment (17.1 ± 0.08 vs. 7.5 ± 2.0 μg/mg dry wt, respectively, p < 0.01). This response to spironolactone was negated by coadministration of aldosterone (hydroxyproline concentration was 18.6 ± 2.1 μg/mg dry wt). Following bilateral adrenalectomy, spironolactone reduced pouch weight and hydroxyproline concentration, which was not the case for adrenalectomy alone. Two week aldosterone administration in uninephrectomized rats on high salt diet was deemed ineffective in modulating pouch development (pouch wet wts were 3.48 ± 0.4 g vs. 3.00 ± 0.19 g in controls and aldosterone-treated rats, respectively). Mineralocorticoid receptor expression in pouch tissue was demonstrated by RT/PCR. Furthermore, NADP+-dependent 11β-hydroxysteroid dehydrogenase 1 (11β-HSD1) activity was detected in pouch tissue, together with lower levels of NAD+-dependent 11β-HSD2. Spironolactone (p < 0.05) significantly reduced 11β-HSD1 activity compared with controls. Thus, fibrous tissue possesses requisite components of MC action, and antagonism of mineralocorticoid receptors by spironolactone attenuates its formation. Pouch formation is under the influence of circulating MC and, we would like to propose, is also mediated through corticosteroids generated de novo at the site of tissue repair.
Similar content being viewed by others
References
Selye H: Use of 'granuloma pouch' technic in the study of antiphlogistic corticoids. Proc Soc Exp Biol Med 82: 328–333, 1953
Selye H: On the mechanism through which hydrocortisone affects the resistance of tissues to injury. JAMA 152: 1207–1213, 1953
Selye H: Interactions between systemic and local stress. Br Med J 4872: 1167–1170, 1954
Weber KT: Glucocorticoids and mineralocorticoids: Antifibrotic and profibrotic components of wound healing. J Lab Clin Med 120: 22–29, 1992
Taubenhaus M, Amromin G: The effects of the hypophysis, thyroid, sex steroids, and the adrenal cortex upon granulation tissue. J Lab Clin Med 36: 7–18, 1950
Trnavsky K, Trnavska Z, Laparova V: Biochemical changes occurring during the development of a turpentine granuloma. Experientia 7: 320–321, 1961
Schiller E: The influence of hormones on the development of silicotic nodules produced by intraperitoneal injection of quartz. Br J Ind Med 10: 1–8, 1953
Rosen H, Blumenthal A, McCallum J: Effect of Asiaticoside on wound healing in the rat. Proc Soc Exp Biol Med 125: 279–280, 1967
Salgado E, Selye H: The role of the adrenals in the production of cardiovascular and renal changes by methylandrostenediol. Arch Int Physiol 62: 352–358, 1954
Weber KT: Hormones and fibrosis: A case for lost reciprocal regulation. News Physiol Sci 9: 123–128, 1994
Hall CE, Hall O: Hypertension and hypersalimentation. 1. Aldosterone hypertension. Lab Invest 14: 285–294, 1965
Brilla CG, Weber KT: Reactive and reparative myocardial fibrosis in arterial hypertension in the rat. Cardiovasc Res 26: 671–677, 1992
Brilla CG, Weber KT: Mineralocorticoid excess, dietary sodium and myocardial fibrosis. J Lab Clin Med 120: 893–901, 1992
Brilla CG, Pick R, Tan LB, Janicki JS, Weber KT: Remodeling of the rat right and left ventricle in experimental hypertension. Circ Res 67: 1355–1364, 1990
Young M, Fullerton M, Dilley R, Funder J: Mineralocorticoids, hypertension, and cardiac fibrosis. J Clin Invest 93: 2578–2583, 1994
Young M, Head G, Funder J: Determinants of cardiac fibrosis in experimental hypermineralocorticoid states. Am J Physiol 269: E657–E662, 1995
Young M: Adrenal steroids and cardiac fibrosis. Steroids 60: 133–136, 1995
Robert V, Van Thiem N, Cheav SL, Mouas C, Swyngliedauw B, Delcayre C: Increased cardiac types I and III collagen mRNAs in aldosterone‐salt hypertension. Hypertension 24: 30–36, 1994
Brilla CG, Zhou G, Matsubara L, Weber KT: Collagen metabolism in cultured adult rat cardiac fibroblasts: Response to angiotensin II and aldosterone. J Mol Cell Cardiol 26: 809–820, 1994
Zhou G, Kandala JC, Tyagi SC, Katwa LC, Weber KT: Effects of angiotensin II and aldosterone on collagen gene expression and protein turnover in cardiac fibroblasts. Mol Cell Biochem 154: 171–178, 1996
Takeda Y, Miyamori I, Yoneda T, Iki K, Hatakeyama H, Blair IA, Hsieh F‐Y, Takeda R: Synthesis of corticosterone in the vascular wall. Endocrinology 135: 2283–2286, 1994
Hatakeyama H, Miyamori I, Fujita T, Takeda Y, Takeda R, Yamamoto H: Vascular aldosterone. Biosynthesis and a link to angiotensin II‐induced hypertrophy of vascular smooth muscle cells. J Biol Chem 269: 24316–24320, 1994
Funder JW, Pearce PT, Smith R, Smith AI: Mineralocorticoid action: Target tissue specificity is enzyme, not receptor, mediated. Science 242: 583–585, 1988
Jenkins JS, Meakin JW, Nelson DH, Thorn GW: Inhibition of adrenal steroid 11‐oxygenation in the dog. Science 128: 478–480, 1958
Randhawa AK, Singal PK: Pressure overload‐induced cardiac hypertrophy with and without dilation. J Am Coll Cardiol 20: 1569–1575, 1992.
Neuman RE, Logan MA: The determination of hydroxyproline. J Biol Chem 184: 299–306, 1950
Slight SH, Ganjam VK, Gómez‐Sánchez CE, Zhou M‐Y, Weber KT: High affinity NAD+‐dependent 11β‐hydroxysteroid dehydrogenase in the human heart. J Mol Cell Cardiol 28: 781–787, 1996
Mishra NC, Estensen RD, Abdel‐Monem MM: Isolation and purification of phorbol from croton oil by reversed‐phase column chromatography. J Chromatogr 369: 435–439, 1986
Sun Y, Ratajska A, Zhou G, Weber KT: Angiotensin converting enzyme and myocardial fibrosis in the rat receiving angiotensin II or aldosterone. J Lab Clin Med 122: 395–403, 1993
Taubenhaus M, Amromin GD: Influence of steroid hormones on granulation tissue. Endocrinology 44: 359–367, 1949
Atkinson RM, Jenkins L, Tomich EG, Woollett EA: The effects of some anti‐inflammatory substances on carrageenin‐induced granulomata. J Endocrinol 25: 87–93, 1962
Ashford A, Penn GB: Effect of adrenalectomy on cotton pellet granuloma formation in the rat. J Pharm Pharmacol 17: 10–14, 1965
Krozowski ZS, Funder JS: Renal mineralocortocoid receptors and hippocampal corticosterone‐binding species have identical intrinsic steroid specificity. Proc Natl Acad Sci USA 80: 6056–6060, 1983
Monder C, White PC: 11β‐hydroxysteroid dehydrogenase. Vit Horm 47: 187–271, 1993
Jamieson PM, Chapman KE, Edwards CRW, Seckl JR: 11β‐hydroxysteroid dehydrogenase is an exclusive 11β‐reductase in primary cultures of rat hepatocytes: Effect of physicochemical and hormonal manipulations. Endocrinology 136: 4754–4761, 1995
Slight S, Ganjam VK, Nonneman DJ, Weber KT: Glucocorticoid metabolism in the cardiac interstitium: 11β‐hydroxysteroid dehydrogenase activity in cardiac fibroblasts. J Lab Clin Med 122: 180–187, 1993
Hammami MM, Siiteri PK: Regulation of 11β‐hydroxysteroid dehydrogenase activity in human skin fibroblasts: Enzymatic modulation of glucocorticoid action. J Clin Endocrinol Metab 73: 326–334, 1991
Klauber N, Browne F, Anand‐Apte B, D'Amato RJ: New activity of spironolactone. Inhibition of angiogenesis in vitro and in vivo. Circulation 94: 2566–2571, 1996
Crum R, Szabo S, Folkman J: A new class of steroids inhibits angiogenesis in the presence of heparin or a heparin fragment. Science 230: 1375–1378, 1985
Folkman J, Weisz PB, Joulli, MM, Li WW, Ewing WR: Control of angiogenesis with synthetic heparin substitutes. Science 243: 1490–1493, 1989
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Slight, S.H., Chilakamarri, V.K., Nasr, S. et al. Inhibition of tissue repair by spironolactone: Role of mineralocorticoids in fibrous tissue formation. Mol Cell Biochem 189, 47–54 (1998). https://doi.org/10.1023/A:1006844010371
Issue Date:
DOI: https://doi.org/10.1023/A:1006844010371