Abstract
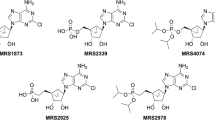
In a previous report, we have demonstrated that simultaneous inhibition of nucleoside transport and adenosine deaminase accumulates endogenous adenosine and protects the myocardium against stunning. The differential cardioprotective effects of erythro-9(2-hydroxy-3-nonyl)-adenine (EHNA), a potent inhibitor of adenosine deamination but not transport, and p-nitrobenzylthioinosine (NBMPR), a selective blocker of adenosine and inosine transport, are not known.
Thirty-seven anaesthetized adult dogs were instrumented to monitor left ventricular performance using sonomicrometery. Dogs were randomly assigned into four groups. The control group (n = 8) received only the vehicle solution. Treated groups received saline containing 100 μM EHNA (EHNA-group, n = 7), 25 μM NBMPR (NBMPR-group, n = 7), or a combination of 100 μM EHNA and 25 μM NBMPR (EHNA/NBMPR-group, n = 10). Hearts were subjected to 30 min of normothermic global ischaemia and 60 min of reperfusion while on bypass. Adenine nucleotides, nucleosides, oxypurines and NAD+ were determined in extracts of transmural myocardial biopsies using HPLC. TTC staining revealed the absence of necrosis in this model.
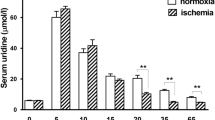
Drug administration did not affect myocardial ATP metabolism and cardiac function in the normal myocardium. Ischemia caused about 50% ATP depletion and accumulation of nucleosides. The ratio between adenosine/inosine at the end of ischemia was 1:10, 1:1, 1:1 and 10:1 in the control, EHNA-, NBMPR- and EHNA/NBMPR-group, respectively. Upon reperfusion, both nucleosides washed out from the myocardium in the control and EHNA-group while retained in the myocardium in the NBMPR and EHNA/NBMPR groups. Ventricular dysfunction 'stunning' persisted in the control group (52%) and in the EHNA-treated group (32%) after 30 min of reperfusion. Significant improvement of function was observed in the EHNA group only after 60 min of reperfusion. LV function recovered in the NBMPR- and EHNA/NBMPR-treated groups during reperfusion. ATP recovery occurred only when animals were pretreated with the combination of EHNA/NBMPR and remained depressed in the control group and EHNA and NBMPR-treated groups. At post mortem, TTC staining revealed the absence of myocardial necrosis.
Superior myocardial protection was observed with inhibition of nucleoside transport by NBMPR alone or in combination with inhibition of adenosine deaminase by EHNA. Selective blockade of nucleoside transport by NBMPR is more cardioprotective than inhibition of adenosine deaminase alone in attenuating myocardial stunning. It is not known why EHNA partially inhibit adenosine deaminase, in vivo.
Similar content being viewed by others
References
Abd-Elfattah AS, Wechsler AS: (eds). In: Purines and Myocardial Protection. Kluwer Academic Publishers, 1996
Abd-Elfattah AS, Wechsler AS: Myocardial protection in cardiac surgery: Subcellular basis for myocardial injury and protection. Adv Cardiac Surg 3: 73–112, 1991
Belardinelli L, Pelleg A: Adenosine and adenine nucleotides: From molecular biology to integrative physiology. Kluwer Academic Publishers, 1995
Lerman BB, Berlardineli L: Cardiac electrophysiology of adenosine: Basic and clinical concepts. Circulation 83: 1499–1509, 1991
Foker JE, Einzig S, Wang T: Adenosine metabolism and myocardial preservation: Consequences of adenosine catabolism on myocardial high-energy compounds and tissue blood flow. J Thorac Cardiovasc Surg 80: 506–516, 1980
Ely SW, Mentzer RM, Lasley RD, Lee BK, Berne RM: Functional and metabolic evidence of enhanced myocardial tolerance to ischemia and reperfusion with adenosine. J Thorac Cardiovasc Surg 90: 549–556, 1990
de Jong WJ, van der Meer P, van Loon H, Owen P, Opie LH: Adenosine as adjunct to potassium cardioplegia: Effect on function, energy metabolism, and electrophysiology. J Thorac Cardiovasc Surg 100: 445–454, 1990
Babbitt OG, Virmani R, Vildibill HD, Norton ED, Forman M: Intracoronary adenosine administration during reperfusion following 3 hours of ischemia: Effect on infarct size, ventricular function, and regional myocardial blood flow. Am Heart J 120: 808–818, 1990
Sollevi A: Clinical studies on the effect of adenosine in role of adenosine and adenine nucleotides in the biological system. In: S Imai, M Nakazawa, (eds). Elsevier Science Publishers, Amsterdam, New York, 1991, pp 525–537
Huizer T, de Jong JW, Nelson JA, Czarnecki W, Serruys PW, Bonnier JJRM, Toquay R: Urate production by human heart. J Mol Cell Cardiol 21: 691–695, 1989
Edlund A, Straat E, Henricksson P: Infusion of adenosine provokes myocardial ischemia in patients with ischemic heart disease. Clin Physiol 9: 309–313, 1989
Sylven C, Jonzon B, Fredholm BB, Kaijser L: Adenosine injection into the brachial artery produces ischemia like pain or discomfort in the forearm. Cardiovasc Res 22: 674–678, 1988
Crea F, El-Tamimi H, Veijar M, Kaski JC, Davies G, Maseri A: Adenosine-induced chest pain in patients with silent and painful myocardial ischemia: Another clue to the importance of generalized defective perception of painful stimuli as a cause of silent ischemia. Eur Heart J 9(suppl N): 34–39, 1988
Ceccareli M, Ciompi ML, Pasero G: Acute renal failure during adenine therapy in the lesch-nyhan syndrome. In: P Sperling, AS De Vries, JB Wyugaarden (eds). Purine Metabolism in Man. Raven Press, New York, 1974, pp 671–679
Ramos-Salazar A, Baines AD: Role of 5'-nucleotidase in adenosine-mediated renal vasoconstriction during hypoxia. J Pharmacol Exp Ther 230: 494–499, 1986
Abd-Elfattah AS, Jessen ME, Lekven J, Doherty NE III, Brunsting LA, Wechsler AS: Myocardial reperfusion injury: Role of myocardial hypoxanthine and xanthine on ‘free-radical mediated reperfusion injury.’ Circulation 1988; 78(suppl III): 224–235
Abd-Elfattah AS, Jessen ME, Hanan SA, Tuchy G, Wechsler AS: Is adenosine-5'-triphosphate derangement or free-radical-mediated injury the major cause of ventricular dysfunction during reperfusion? Role of adenine nucleoside transport in myocardial reperfusion injury. Circulation; 82(suppl IV): IV-341–350, 1990
Abd-Elfattah AS, Ding M, Wechsler AS: Protection of the stunned myocardium: Selective nucleoside transport blocker (NBMPR) administered after 20 min of ischemia augments recovery of ventricular function. Circulation; 88 [part 2]: 336–343, 1993
Abd-Elfattah AS, Jessen ME, Wechsler AS: Nucleoside Trapping during reperfusion prevents ventricular dysfunction ‘stunning’ in absence of adenosine: Possible separation between ischemic and reperfusion injury. J Thorac Cardiovasc Surg 108: 269–278, 1994
Abd-Elfattah AS, Wechsler AS: Separation between ischemic and reperfusion injury by site specific entrapment of endogenous adenosine and inosine using NBMPR and EHNA. J Cardiac Surgery 9 (suppl): 387–396, 1994
Abd-Elfattah ASA, Wechsler AS: Nucleoside transport and myocardial injury and protection: Possible separation between ischemic and reperfusion injury by selective nucleoside transport blocker NBMPR. In: AS Abd-Elfattah, AS Wechsler (eds). Purines and Myocardial Protection. Kluwer Academic Publishers, 1995
Abd-Elfattah AS, Ding M, Wechsler AS: Intermittent warm aortic cross-clamping prevents cumulative ATP depletion, ventricular fbrillation and dysfunction ‘stunning’: Is it preconditioning? J Thorac Cardiovasc Surg 110: 328–339, 1995
Abd-Elfattah ASA, Maddox R, Jessen ME, Hanan SA, Wechsler AS: Selective nucleoside transport blocker NBMPR attenuates myocardial stunning in a rabbit model-defcient of xanthine oxidase. In: AS Abd-Elfattah, AS Wechsler (eds). Purines and Myocardial Protection, Kluwer Academic Publishers, 1995
Dow JW: Metabolism of purines by adult cardiomyocytes. In: HM Piper, G Isenberg, (eds). Isolated adult cardiomyocytes. 1989, pp 216–237
Jennings RB, Reimer KA, Hill ML, et al.: Total ischemia, in dogs hearts, in vitro. I. Comparison of high energy phosphate production, utilization and depletion and adenine nucleotide catabolism in total ischemia in vitro vs. severe ischemia in vivo. Circ Res 49: 892–900, 1981
Jarasch ED, Bruder G, Heid HW: Significance of xanthine oxidase in capillary endothelial cells. Acta Physiol Scand 548(suppl): 39–46, 1986
Chambers DE, Parks DA, Patterson G, et al.: Xanthine oxidase as a source of free radical damage in myocardial ischemia. J Mol Cell Cardiol 17: 145–152, 1985
Eddy LJ, Stewart JR, Jones HP, Engerson TD, McCord JM, Downey JM: Free radical producing enzyme, xanthine oxidase is undetectable in human hearts. Am J Physiol 253: H709–H711, 1987
Muxfeldt M, Schaper W: The activity of xanthine oxidase in hearts of pigs, guinea pigs, rats and humans. Basic Res Cardiol 82: 486–492, 1987
Abd-Elfattah AS, Salter DR, Murphy CE, Goldstein JP, Brunsting LA, Wechsler AS: Metabolic differences between retrograde and antegrade cardioplegia after reversible normothermic global ischemic injury. Surg Forum 37: 267–270, 1986
Bolli R. Oxygen-derived free eadiacals and myocardial reperfusion injury. An overview. Cardiovasc Drug Ther 5: 225–236, 1991
Zwier JL. Measurement of superoxide-derived free radicals inthe reperfused heart: Evidence for a free radical mechanism of reperfusion injury. J Biol Chem 263: 1353–1357, 1988
Horwitz LD, Fennessey PV, Shikesss RH, Kong Y. Marked reduction in myocardial infarct size due to prolonged infusion of an antioxidant during reperfusion. Circulation 89: 1792–1801, 1994
Akizuki S, Yoshida, Chamber DE, Eddy LJ, Paaarmley LF, Yellon DM, Downey JM. Infarct size limitation by the xanthine oxidase inhibitor allopurinol, in closed chest dogs with small infarcts. Cardiovasc Res 19: 686–692, 1985
Glower DD, Spratt JA, Snow ND, Kabas JS, Davis JW, Olsen CO, Tyson GS, Sabiston DC, Rankin JS: Linearity of the Frank-Starling relationship in the intact heart: The concept of preload recruitable stroke work. Circulation 71: 994–1009, 1985
Spratt JA, Tyson GS, Glower DD, Davis JW, Muhlbair LH, Olsen CO, Rankin JS: The end systolic pressure-volume relationship in conscious dogs. Circulation 75: 1295–1309, 1987
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ: Protein measurement with Folin phenol reagent. J Biol Chem 193: 265–275, 1951
Abd-Elfattah AS, Wechsler AS: Superiority of HPLC to assay for enzymes regulating adenine nucleotidase pool intermediates metabolism: 5'-nucleotidase, adenylate deaminase, adenosine deaminase and adenylosuccinate lyase: A simple and rapid determination of adenosine. J Liquid Chromatogr 10: 2653–2694, 1987
Lamb CU, Nelson DJ: Pharmacokinetics of inhibition of adenosine deaminase by erythro-9 (2-hyroxy-3-nonyl) adenine in CBA mice. Biochem Pharmacol 31: 535–539, 1982
Van der Hied R, Reimer KA, Jennings RB: Adenosine slow ischemic metabolism in canine myocardium in vitro: Relationship to ischemic preonditioning. Cardiovasc Res 27: 669–673, 1993
Abd-Elfattah ASA, Guo JH, El-Quessaab E, Goa SP, Mahgoub M: Failure of adenosine deaminase inhibitors (+)EHNA, PVP-27, PVP-30 and VMV to favorably accumnalte adenosine and protect against myocardial ischemia. J Mol Cell Cardiol; 28: (abstr) A182, 1996
Vargeese C, Sarma MSP, Pragacharyulu PVP et al.: Adenosine deaminase inhibitors. Synthesis and biological evaluation of putative metabolises of (+)-erythro-9-(2S-hydroxy-3R nonyl)adenine. J Med Chem 37: 3844–3849, 1994
Van Wylen DGL, Willis J, Sodhi J, Weiss RJ, Lasley RD, Mentzer RM Jr: Cardiac microdialysis to estimate regional interstitial adenosine concentration and coronary blood flow. Am J Physiol 258: H1642–H1649, 1990
Van-Wylen DGL: Assessment of interstitial fluid adenosine: Implication for exogenous and endogenous adenosine-mediated cardioprotection. In: AS Abd-Elfattah, AS Wechsler (eds). Purines and Myocardial Protection. Kluwer Academic Publishers, 1996, pp 81–94
Wang T, Mentzer RM Jr, Van Wylen DG: Interstitial adenosine with dipyridamole: Effect of adenosine receptor blockade and adenosine deaminase inhibition. Am J Physiol 263: H552–H558, 1992
Tibi PR, Patti CS, Pearce FJ, Hicks GL: Deleterious effects of dipyridamole during reperfusion of ischemic myocardium. Surgery 36: 255–258, 1985
Takeo S, Tanonaka K, Tazuma Y, Fukao N, Yoshikawa C, Fukumoto T, Tanaka T: Diltiazem and verapamil reduce the loss of adenine nucleotide metabolites from hypoxic hearts. J Mol Cell Cardiol 1988: 20(5): 443–456, 1988
Lesnefsky EJ, Van Benthuysen KM, McMurtry IF, Shikes RH, Johnston RB Jr, Horwitz LD: Lidocaine reduces canine infarct size and decreases release of a lipid peroxidation product. J Cardiovasc Pharmacol 13: 895–901, 1989
Flameng W, Dacnen W, gorgers M, Thone F, Xhonneux R, van de Waters A, Van Belle H: Cardioprotective effects of lidoflazine during lh of normothermic global ischemia. Circulation 64: 796–807, 1981
Gruber HE, Hoffer MF, McAllister DR, Laikind PK, Lame TA, Schmid-Schoenbin GW, Engler RL: Increased adenosine concentration in blood from ischemic myocardium by AICA riboside: Effects on flow, granulocytes and injury. Circulation 80: 1400–1411, 1989
Grover GT, Sleph PG: The cadrioprotective effects of R 75231 and lidoflazin are not caused by adenosine receptor activation. J Pharmacol Exp Therap 268: 90–96, 1994... related to Ca2+
Flameng W, Xhonneux R, Van Belle H, van de Waters A, Wouters L, Wijoants J, Thone' F, Van Deale P, Janssen PA: Cardioprotective effects of mioflazine during 1h normothermic global ischemia in the canine heart. Cardiovasc Res 18: 528–537, 1984
Gorgers M, et al.: Pathophysiology of cardiomyocytes. Ann NY Acad Sci 5: 433–453, 1988
Buchwald A, Ito BR, Schaper W: Influence of mioflazine on canine coronary blood flow and on adenine nucleotide and nucleoside content under normal and ischemic conditions. J Cardiovasc Pharmacol 10: 213–221, 1987
Paterson ARP, Jakobs ES, Ng CYC, Odegard RD, Adjoi AA: Nucleoside transport inhibition in vitro and in vivo. In: E Gerlach, BF Becker (eds). Topics and Perspectives in Adenosine Research. Springer-Verlag, Berlin, 1987, pp 89–101
Zughaib ME, Abd-Elfattah AS, Jeroudi MO, Sun J-Z, Sekili S, Tang X-L, Bolli R: Inhibitors of adenosine deaminase and nucleoside transport attenuate myocardial ‘stunning’ independently of coronary flow or hemodynamic effects. Circulation 88 [part 1]: 2359–2369, 1993
Hearse DJ, Garlick PB, Humphrey SP: Ischemia contracture of the myocardium: Mechanisms and prevention. Am J Cardiol 39: 986–993, 1977
Glower DD, Spratt JA, Newton JR, Wolf JA, Rankin JS, Swain JL: Dissociation between early recovery of regional function and purine nucleotidase content in postischemic myocardium in conscious dogs. Cardiovasc Res 21: 328–336, 1987
Hearse DJ: Oxygen derivation and early myocardial contractile failure: A reassessment of the role of adenosine triphosphate. Am J Cardiol 44: 1115–1121, 1979
Grum CM, Ketai LH, Myers CL, Shlafer M: Purine efflux after cardiac ischemia: Relevance to allopurinol cardioprotection. Am J Physiol 252: H368–H373, 1987
Fox AC, Reed GE, Meilman H, Silk BB: Release of nucleoside from canine and human hearts as an index of prior ischemia. Am J Cardiol 43: 52–58, 1979
Wyatt DA, Edmunds MC, Ribuo R, Berne RM, Lasley RD, Mentzer RM: Adenosine stimulates glycolytic flux in isolated perfused rat heart by α1-adenosine receptors. Am J Physiol 257: H1952–H1957, 1989
Lasley RD, Mentzer RM: Adenosine improves recovery of postischemic myocardial function via an adenosine A, receptor mechanism. Am J Physiol 263: H1460–H1465, 1992
Liu GS, Thornton J, Van Winkle DM, Staney AWH, Olsson RA, Downey JM: Protection against infraction afforded by preconditioning is mediated by A1 adenosine receptors in rabbit heart. Circulation 84: 350–356, 1991
Cave AC, Downey JM, Hearse DJ: Adenosine fails to substitute for preconditioning in the globally ischemic isolated rat heart. J Mol Cell Cardiol 1991; 23: (abst) P-3–30
Hendrikx M, Toshima, Mubagwa K, Flameng W: Improved functional recovery after ischemic preconditioning in the globally ischemic rabbit heart is not mediated by A1 receptor activation. Circulation 1992; 86(spl I): I–342, (abstr) 1992
Abd-Elfattah AS, Ding M, Wechsler AS: Myocardial stunning and preconditioning: Age-, species-, and model-related differences: Role of AMP-5'-nucleotidase in myocardial injury and protection. J Cardiac Surg 199(35): 101–105
Abd-Elfattah AS, Ding M, Nomier FR, Mustafa SJ, Mansour MA, Wechsler AS: A1-receptor blockade augments postischemic ventricular dysfunction ‘stunning’ in the presence or absence of endogenous adenosine. Circulation (suppl I); 88:I–131, (abstr) 1993
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Abd-Elfattah, A.S., Jessen, M.E., Lekven, J. et al. Differential cardioprotection with selective inhibitors of adenosine metabolism and transport: Role of purine release in ischemic and reperfusion injury. Mol Cell Biochem 180, 179–191 (1998). https://doi.org/10.1023/A:1006828115191
Issue Date:
DOI: https://doi.org/10.1023/A:1006828115191