Abstract
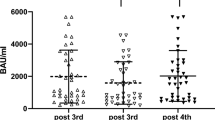
Seven individuals with late complement component (LCC) deficiency and seven control subjects were vaccinated with tetravalent meningococcal vaccine. The response to vaccination was evaluated by measuring the antibody titer and the phagocyte killing of the bacteria, before, 5–7 weeks, and 12–14 months after vaccination. Prior to vaccination, no phagocytic killing and a low titer of antibody was found in the LCC-deficient group and a low killing (mean of 40–58%, according to the serogroup) in normal controls. The phagocytic killing increased significantly 5–7 weeks after vaccination. However, while in normal controls the phagocytic killing was close to 100% after 5–7 weeks and decreased only slightly during the first year, the mean killing of the various meningococcal subgroups in LCC-deficient individuals was 70–89% and dropped to only 53–71% one year after vaccination. Six weeks after vaccination the mean antimeningococcal antibody titer increased similarly in the sera of LCC-deficient patients and controls. One year after vaccination the controls maintained the high concentration, while the LCC-deficient patients had tendency toward a decrease. In addition, the interpersonal variability of the antibody concentration, both in LCC-deficient individuals and in normal controls, was much higher than the phagocytic killing, with only a very mild increase in some individuals. Thus, it is possible that in spite of adequate increase of antimeningococcal antibody titer after vaccination of LCC-deficient individuals their immunity against the bacteria may not be optimal. Our data show also that phagocytic killing of meningococci is probably a more consistent assay than antibody titer levels for antimeningococcal immunity, especially in LCC-deficient patients.
Similar content being viewed by others
REFERENCES
Roberts RB: The relationship between group A and group C meningococcal polysaccharides and serum opsonins in man. J Exp Med 131:409-513, 1970
Ross SC, Rosentha PY, Densen P: Killing of Neisseria meningitidis by human neutrophils: Implications for normal and complement deficient individuals. J Infect Dis 126:1266-1275, 1987
Biselli R, Casapollo I, D'Amelio R, Salvato S, Marticardi PM, Brai M: Antibody response to meningococcal polysaccharide A and C in patients with complement defects. Scand J Immunol 37:644-650, 1993
Schlesinger M, Greenberg M, Levy J, Kayhty H, Levy R: Killing of meningococci by neutrophils—Effect of vaccination in patients with complement deficiency. J Inf Dis 170:449-453, 1994
Andreoni J, Kayhty H, Densen P: Vaccination and the role of capsular polysaccharide antibody in prevention of recurrent meningococcal disease in late complement component-deficient individuals. J Inf Dis 168:227-231, 1993
Platonov AE, Beloborodov VB, Pavlova LI, Vershinina IV, Kayhty H: Vaccination of patients deficient in a late complement component with tetravalent meningococcal capsular polysaccharide vaccine. Clin Exp Immunol 100:32-39, 1995
Levy R, Klein J, Rubinek T, Alkan M, Shany S, Chaimovitz C: Diversity in peritoneal macrophages of CAPD patients to 1.25 dihydroxyvitamin D3. Kidney Int 37:310-315, 1990
Ross SC, Densen P: Opsonophagocytosis of Neisseria gonorrhea interaction of local and disseminated isolates with complement and neutrophils. J Infect Dis 15:33-41, 1985
Arakere G, Frasch CE: Specificity of antibodies to O-acetylpositive and O-acetyl-negative group C meningococcal polysaccharides in sera from vaccines and carriers. Inf Imm 59:4349-4356, 1991
Holder PK, Maslanka SE, Pais LB, Dykes J, Plikaytis BD, Carlone GM: Assignment of Neisseria meningitidis serogroup A and C class-specific anticapsular antibody concentration to the new standard reference serum CDC1992. Clin Diag Lab Immunol 2:132-137, 1995
Ross SC, Densen P: Congenital deficiency states and infections. Epidemiology, pathogenesis and consequences of neisserial and other infections in an immune deficiency. Medicine 63:243-273, 1984
Densen P: Complement deficiencies and meningococcal disease. Clin Exp Immunol 86:(Suppl 1) 57-62, 1991
Wurzner R, Oren A, Lacman PJ: Inherited deficiencies of the terminal components of human complement. Immunodeficiency Reviews 3:123-147, 1992
Potter PC, Fresch CE, van-der-Sande WJ, Cooper RC, Patel Y, Orren A: Prophylaxis against Neisseria meningitidis infections and antibody response in patients with deficiency of the sixth component of complement. J Infect Dis 161:932-937, 1990
Densen P, Brown EJ, O'Neill GJ, Tedesco F, Clark RA, Frank MM, Webb D, Myers J: Inherited deficiency of C8 in a patient with recurrent meningococcal infections: further evidence for a dysfunctional C8 molecule and non-linkage to the HLA system. J Clin Immunol 3:90-99, 1983
Meningitis and Special Pathogens Branch, Division of Bacterial Diseases, Center for Infectioious Disease, CDC: Availability of meningococcal vaccine in single-dose for travellers and high risk persons. MMWR 39:763, 1990
Platonov AE, Vershinia IV, Dankert J, Kuijper EJ, Gustafson L, Kayhty H: Long-term follow-up of late complement component deficient patients vaccinated with meningococcal polysaccharide vaccine: Antibody persistence and efficacy of vaccination (Abst.). Tenth International Pathogenic Neisseria Conference, Baltimore, Maryland, USA, September 1996
Fijen CAP, Kuijper EJ, Drogari-Apiranthitou M, Van Leeuwen Y, Daha MR, Dankert J: Protection against meningococcal serogroup ACYW disease in complement-deficient individuals vaccinated with the tetravalent meningococcal capsular polysacharide vaccine. Clin Exp Immunol 114:362-369, 1998
Orren A, Warren RE, Potter PC, Jones AM, Lachmann PJ, Poolman JT: Antibodies to meningococcal class 1 outer membrane proteins in south Africa complement-deficient and complement-sufficient subjects. Infect Immun 60:4510-4516, 1992
King WJ, MacDonald NE, Wells G, Allen U, Chan F, Ferris W, Diaz-Mitoma F, Ashton F: Total and functional antibody response to a quadrivalent meningococcal polysaccharide vaccine among children. J Pediatr 128:196-202, 1996
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schlesinger, M., Kayhty, H., Levy, R. et al. Phagocytic Killing and Antibody Response During the First Year After Tetravalent Meningococcal Vaccine in Complement-Deficient and in Normal Individuals. J Clin Immunol 20, 46–53 (2000). https://doi.org/10.1023/A:1006642611069
Issue Date:
DOI: https://doi.org/10.1023/A:1006642611069