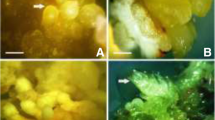
Abstract
Somatic embryogenesis was achieved from callus, cell suspension and protoplast culture systems in the endemic black iris (Iris nigricans). Subculture of friable callus fragments on embryogenesis induction medium (EIM) containing 4.5 μM 2,4-dichlorophenoxyacetic acid (2,4-D), 0.5 μM kinetin, 4.5 μM 1-naphthaleneacetic acid (NAA) and 300 mg l-1 proline in the dark was necessary before transfer to regeneration medium (RM). Regeneration was studied by transferring friable callus fragments from EIM to RM containing (0.0, 4.5, 9.0, 13.5 μM) of either 6-benzyladenine (BA), 2-isopentenyladenine (2iP), zeatin or thidiazuron (TDZ) in combination with 0.49 μM indole-3-butyric acid (IBA), 0.45 μM 2,4-D. Maximum embryogenesis was obtained at 4.5 μM BA while zeatin and TDZ were not effective and embryogenesis did not occur with these treatments. Sucrose at 0.2 M was more effective for embryogenesis when compared to glucose or fructose. Growing cells in suspension culture on EIM containing 4.5 μM 2,4-D in combination with 0.2 M sucrose for four weeks and transferring cells to RM (containing 4.5 μM BA) gave significant embryogenesis with maximum number of embryos (3568 embryos/g cells). Using 4.5 μM 2,4-D in protoplast culture was necessary for the best protoplast division and colony formation. In all experiments, embryos developed on RM were transferred to hormone-free medium (HFM) and 90% converted to rooted plantlets. Produced plantlets gave 95% survival ex vitro. Plantlets developed to whole plants in the greenhouse and flowered.
Similar content being viewed by others
References
Anderson WC, Mielke KA, Miller PN & Allen T (1992) In vitro propagation of virus-free Dutch iris. Acta Hort. 266: 77–81
Bertaccini A & Bellardi MG (1991) Shoot-tip culture in the production of virus-indexed Dutch iris. Adv. Hort. Sci. 5: 23–26
Derks AFLM, Hollinger THC & van Vink JLDAL (1985) Identification and symptoms expression of four elongated viruses infecting bulbous irises. Acta Hort. 164: 309–317
Gozu Y, Yokoyama M, Nakamura M, Namba R, Yomogida K, Yanagi M & Nakamura S (1993) In vitro propagation of Iris pallida. Plant Cell Rep. 13: 12–16
Jehan H, Courtois D, Ehret C, Lerch K & Petiard V (1994) Plant regeneration of Iris pallida Lam. and Iris germanica L. via somatic embryogenesis from leaves, apices and young flowers. Plant Cell Rep. 13: 671–675
Laublin G & Cappadocia M (1990) Plant regeneration via embryogenesis in several Iris species. In: Proc. Intern. Congr. Plant Tiss. Cell Cult. Amsterdam, Abstr. A3–43
Laublin G, Saini HS & Cappadocia M (1991) In vitro plant regeneration via somatic embryogenesis from root culture of some rhizomatous irises. Plant Cell Tiss. Org. Cult. 27: 15–21
Murashige T & Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15: 473–479
Radojevic L & Subotic A (1992) Plant regeneration of Iris setosa Pall. through somatic embryogenesis and organogenesis. J. Plant Physiol. 139: 690–696
Radojevic L, Sokic O & Tucic B (1987) Somatic embryogenesis in tissue culture of iris (Iris pumila L.). Acta Hortic. 212: 719–723
Reuther G (1977) Adventitious organ formation and somatic embryogenesis in callus of asparagus and iris and its possible applications. Acta Hort. 78: 217–224
SAS (1989) Version 6, 4th ed. SAS Institute, Inc, Cary NC, USA
Shibli RA (2000) Cryopreservation of black iris (Iris nigricans) somatic embryos by encapsulation – dehydration. Cryo-Lett. 21: 39–46
Shibli RA, Smith MAL & Shatnawi M (1999) Pigment recovery from encapsulated-dehydrated Vaccinium pahalae (ohelo) cryopreserved cells. Plant Cell Tiss. Org. Cult. 55: 119–123
Shimizu K, Nagaike H & Adachi T (1997) Plant regeneration from suspension culture of Iris germanica. Plant Cell Tiss. Org. Cult. 50: 27–31
Shimizu K, Yabuya T & Adachi T (1996) Plant regeneration from protoplast of Iris germanica L. Euphytica. 89: 223–227
Tsukahara M, Hirosawa T & Kishine S (1996) Efficient plant regeneration from cell suspension of rice (Oryza sativa L.). J. Plant Physiol. 149: 157–162
Van der Linde PCG, Hol GMGM, Blom-Barnhoorn GJ, van Aartijk J & de Klerk GJ (1988) In vitro propagation of Iris hollandica Tub. CV. Prof. Blaauw. I. Regeneration on bulb-scale explants. Acta Hortic. 226: 121–128
Wang X, Jeknic Z, Ernst RC & Chen THH (1999). Efficient plant regeneration from suspension-cultured cells of tall bearded iris. HortScience 34: 730–735
Yabuya I, Y Ikeda & Adachi T (1991) In vitro propagation of Japanese garden iris, Iris ensata Thunb. Euphytica 57: 77–81
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shibli, R.A., Ajlouni, M. Somatic embryogenesis in the endemic black iris. Plant Cell, Tissue and Organ Culture 61, 15–21 (2000). https://doi.org/10.1023/A:1006468122819
Issue Date:
DOI: https://doi.org/10.1023/A:1006468122819