Abstract
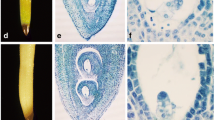
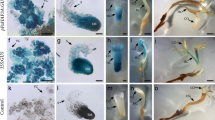
PsMAPK3, a new MAP kinase cDNA, was cloned from ovaries of Pisum sativum L. Expression of PsMAPK3 is at low basal levels in unpollinated ovaries but it is rapidly induced by gibberellic acid (peak at 30 min) and 6-benzyladenine (peak at 45 min). Both treatments promoted the development of a parthenocarpic fruit. In situ hybridization localized PsMAPK3 mRNA in ovules. The transcript was additionally detected in the mesocarp when it is expanding in response to the treatments. These observations suggest that gibberellins and cytokinins regulate PsMAPK3 mRNA levels in pea ovary shortly after fruit set is induced.
Similar content being viewed by others
References
Alabadí, D. and Carbonell, J. 1999. Differential expression of two spermidine synthase genes during early fruit development and in vegetative tissues of pea. Plant Mol. Biol. 39: 933–943.
Bögre, L., Ligterink, W., Heberle-Bors, E. and Hirt, H. 1996. Mechanosensors in plants. Nature 383: 489–490.
Bögre, L., Ligterink, W., Meskiene, I., Barker, P.J., Heberle-Bors E., Huskisson, N.S. and Hirt, H. 1997. Wounding induces the rapid and transient activation of a specific MAP kinase pathway. Plant Cell 9: 75–83.
Carbonell, J. and García-Martínez, J.L. 1985. Ribulose-1,5-bisphosphate carboxylase and fruit set or degeneration of unpollinated ovaries of Pisum sativum L. Planta 164: 534–539.
Cercós, M., Santamaría, S. and Carbonell, J. 1999. Cloning and characterization of TPE4A, a thiol protease gene induced during ovary senescence and seed germination in pea. Plant Physiol. 119: 1341–1348.
Decroocq-Ferrant, V., Decroocq, S., van Went, J., Schmidt, S. and Kreis, M. 1995. A homologue of the MAPK/ERK family of protein kinase genes is expressed in vegetative and in female reproductive organs of Petunia hybrida. Plant Mol. Biol. 27: 339–350.
Devereux, J., Haeberli, P. and Smithies, O. 1984. A comprehensive set of sequence analysis programs for the VAX. Nucl. Acids Res. 12: 387–395.
Frohman, M.A., Dush, M.K. and Martin, G.R. 1988. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc. Natl. Acad. Sci. USA 85: 8998–9002.
García-Martínez, J.L. and Carbonell, J. 1980. Fruit-set of unpollinated ovaries of Pisum sativum L. Influence of plant-growth regulators. Planta 147: 451–456.
García-Martínez, J.L. and Hedden, P. 1997. Gibberellins and fruit development. In: F.A. Tomás-Barberán and R.J. Robins (Eds.) Phytochemistry of Fruit and Vegetables, Clarendon Press, Oxford, pp. 263–285.
Gillaspy, G., Ben-David, H. and Gruissem, W. 1993. Fruits: a developmental perspective. Plant Cell 5: 1439–1451.
Gómez-Gómez, L. and Carrasco, P. 1996. Hormonal regulation of S-adenosylmethionine synthase transcripts in pea ovaries. Plant Mol. Biol. 30: 821–832.
Granell, A., Harris, N., Pisabarro, A.G. and Carbonell, J. 1992. Temporal and spatial expression of a thiol protease gene during 186 pea ovary senescence and its regulation by gibberellin. Plant J. 2: 907–915.
Hanks, S.K., Quinn, A.M. and Hunter, T. 1988. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science 241: 42–52.
Huttly, A.K. and Phillips, A.L. 1995. Gibberellin-regulated expression in oat aleurone cells of two kinases that show homology to MAP kinase and a ribosomal protein kinase. Plant Mol. Biol. 27: 1043–1052.
Jackson, D.P. 1992. In situ hybridization in plants. In: D.J. Bowles, S.J. Gurr and M. Pherenson (Eds.) Molecular Plant Pathology: A Practical Approach, Oxford University Press, Oxford, UK, pp. 163–174.
Jahnke, S., Bier, D., Estruch, J.J. and Beltrán, J.P. 1989. Distribution of photoassimilates in the pea plant: chronology of events in nonfertilized ovaries and effects of gibberellic acid. Planta 180: 53–60.
Jonak, C., Kiegerl, S., Ligterink, W., Barker, P.J., Huskisson, N.S. and Hirt, H. 1996. Stress signaling in plants: a mitogen-activated protein kinase pathway is activated by cold and drought. Proc. Natl. Acad. Sci. USA 93: 11274–11279.
Jonak, C., Ligterink, W. and Hirt, H. 1999. MAP kinases in plant signal transduction. Cell Mol. Life Sci. 55: 204–213.
Joshi, C.P., Zhou, H., Huang, X. and Chiang, V.L. 1997. Context sequences of translation initiation codon in plants. Plant Mol. Biol. 35: 993–1001.
Kieber, J.J., Rothenberg, M., Roman, G., Feldmann, K.A. and Ecker, J.R. 1993. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of protein kinases. Cell 72: 427–441.
Knetsch, M., Wang, M., Snaarjagalska, B.E., Heimovaara-Dijkstra, S. 1996. Abscisic acid induces mitogen-activated protein kinase activation in barley aleurone protoplasts. Plant Cell 8: 1061–1067.
Kovtun, Y., Chiu, W.L., Zeng, W.K. and Sheen, J. 1998. Suppression of auxin signal transduction by a MAPK cascade in higher plants. Nature 395: 716–720.
Ligterink, W., Kroj, T., zur Nieden, U., Hirt, H. and Scheel, D. 1997. Receptor-mediated activation of a MAP kinase in pathogen defense of plants. Science 276: 2054–2057.
Mizoguchi, T., Hayashida, N., Yamaguchi-Shinozaki, K., Kamada, H. and Shinozaki, K. 1993. ATMPKs: a gene family of plant MAP kinases in Arabidopsis thaliana. FEBS Lett. 336: 440–444.
Mizoguchi, U., Irie, K., Hirayama, T., Hayashida, N., Yamaguchi-Shinozaki, K., Matsumoto, K. and Shinozaki, K. 1996. A gene encoding a mitogen-activated protein kinase kinase kinase is induced simultaneously with genes for a mitogen-activated protein kinase and an S6 ribosomal protein kinase by touch, cold and water stress in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 93: 765–769.
Mizoguchi, T., Ichimura, K., Yoshida, R. and Shinozaki, K. 2000. MAP kinase cascades in Arabidopsis: their roles in stress and hormone responses. In: H. Hirt (Ed.) Results and Problems in Cell Differentiation Vol. 27, Springer-Verlag, Berlin/Heidelberg, pp. 29–38.
Orzáez, D., Blay, R. and Granell, A. 1999. Program of senescence in petals and carpels of Pisum sativum L. flowers and its control by ethylene. Planta 208: 220–226.
Pereto, J.G. and Beltran, J.P. 1987. Hormone directed sucrose transport during fruit set induced by gibberellins in Pisum sativum. Physiol. Plant. 69: 356–360.
Pérez-Amador, M.A. and Carbonell, J. 1995. Arginine decarboxylase and putrescine oxidase in ovaries of Pisum sativum L. Changes during ovary senescence and early stages of fruit development. Plant Physiol. 107: 865–872.
Popping, B., Gibbons, T. and Watson, M.D. 1996. The Pisum sativum MAP kinase homologue (PsMAPK) rescues the Saccharomyces cerevisiae hog1 deletion mutant under conditions of high osmotic stress. Plant Mol. Biol. 31: 355–363.
Robinson, M.J. and Cobb, M.H. 1997. Mitogen-activated protein kinase pathways. Curr. Opin. Cell Biol. 9: 180–186.
Rodríguez-Concepción, M. and Beltrán, J.P. 1995. Repression of the pea lipoxygenase gene loxg is associated with carpel development. Plant Mol. Biol. 27: 887–899.
Romeis, T., Piedras, P., Zhang, S.Q., Klessig, D.F., Hirt, H. and Jones, J. 1999. Rapid Avr9-and Cf-9-dependent activation of MAP kinases in tobacco cell cultures and leaves: convergence of resistance gene, elicitor, wound, and salicylate responses. Plant Cell 11: 273–287.
Sambrook, J., Fritsch, E.F. and Maniatis, T. 1989. Molecular Cloning: A Laboratory Manual, 2nd ed., Cold Spring Harbor Laboratory Press, Plainview, NY.
Sánchez-Beltrán, M.J., Carbonell, J., García-Martínez, J.L. and López-Díaz, I. 1992. Gene expression during two alternative pathways of ovary development in Pisum sativum: fruit development and ovary senescence. Physiol. Plant. 85: 476–482.
Seo, S., Okamoto, N., Seto, H., Ishizuka, K., Sano, H. and Ohashi, Y. 1995. Tobacco MAP kinase: a possible mediator in wound signal transduction pathways. Science 270: 1988–1992.
Seo, S., Sano, H. and Ohashi, Y. 1999. Jasmonate-based wound signal transduction requires activation of WIPK, a tobacco mitogen-activated protein kinase. Plant Cell 11: 289–298.
Stafstrom, J.P., Altschuler, M. and Anderson, D.H. 1993. Molecular cloning and expression of a MAP kinase homologue from pea. Plant Mol. Biol. 22: 83–90.
Tena, G. and Renaudin, J.P. 1998. Cytosolic acidification but not auxin at physiological concentration is an activator of MAP kinases in tobacco cells. Plant J. 16: 173–182.
Vercher, Y., Molowny, A., López, C., García-Martínez, J.L. and Carbonell, J. 1984. Structural changes in the ovary of Pisum sativum L. induced by pollination and gibberellic acid. Plant Sci. Lett. 36: 87–91.
Vercher, Y. and Carbonell, J. 1991. Changes in the structure of ovary tissues and in the ultrastructure of mesocarp cells during ovary senescence of fruit development induced by plant growth substances in Pisum sativum. Physiol. Plant. 81: 518–526.
Zhang, S. and Klessig, D.F. 1998a. The tobacco woundingactivating MAP kinase is encoded by SIPK. Proc. Natl. Acad. Sci. USA 95: 7225–7230.
Zhang, S. and Klessig, D.F. 1998b. Resistance gene N-mediated de novo synthesis and activation of a tobacco mitogen-activated protein kinase by tobacco mosaic virus infection. Proc. Natl. Acad. Sci. USA 95: 7433–7438.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Marcote, M.J., Carbonell, J. Transient expression of a pea MAP kinase gene induced by gibberellic acid and 6-benzyladenine in unpollinated pea ovaries. Plant Mol Biol 44, 177–186 (2000). https://doi.org/10.1023/A:1006434330381
Issue Date:
DOI: https://doi.org/10.1023/A:1006434330381