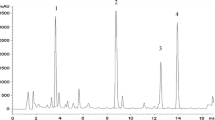
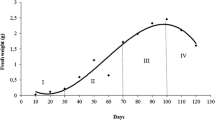
Abstract
Six different callus lines and three different suspension culture lines were established from plants of two Aphelandra species (Acanthaceae). All established lines were analyzed for secondary metabolite accumulation. A discrepancy between secondary metabolites accumulated in the plants and in the cell cultures could be observed. All established Aphelandrasp. cell cultures produced verbascoside (acteoside) as the major extractable metabolite. Time course experiments were carried out to investigate the relationship between cell growth and verbascoside production. In the present study it was shown that verbascoside accumulation was growth dependent and positively related to the presence of 2,4-D in the medium. The conditions in which verbascoside represents ca. 18% of cell culture weight have been defined. Free polyamines were detected in the cell culture lines cultivated in MS liquid medium (cysteine 10 mg l-1, thiamine 1 mg l-1, 2,4-D 1 mg l-1, kinetin 0.2 mg l-1 and sucrose 30 g l-1). Putrescine and spermidine accumulated within 8 days to a maximum of 8.4 μmol g-1 of dry wt and 2.6 μmol g-1 of dry wt respectively and thereafter their concentration decreased rapidly. There was no evidence for the presence of spermine or any other type of free or conjugated polyamines in the tested cell culture lines.
Similar content being viewed by others
References
Andary C, Wylde R, Laffite C, Privat G & Winternitz F (1982) Structures of verbascoside and orobanchoside, caffeic acid sugar esters from Orobanche rapum-genistae. Phytochemistry 21: 1123–1127
Dell B, Elsegood CL & Ghisalberti EL (1989) Production of verbascoside in callus tissue of Eremophila spp. Phytochemistry 28: 1871–1872
Ellis BE (1983) Production of hydroxyphenylethanol glycosides in suspension cultures of Syringa vulgaris. Phytochemistry 22: 1941–1943
Fowler MW, Cresswell RC & Stafford AM (1990) An economic and technical assessment of the use of plant cell cultures for natural product synthesis on an industrial scale. In: Chadwick DJ & Marsh J (ed) Bioactive Compounds from Plants, Ciba Foundation Symposium, Vol 154(pp 157–174). John Wiley & Sons Ltd.
Gundlach H, Müller MJ, Kutchan TM & Zenk MH (1992) Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proc. Natl. Acad. Sci. USA 89: 2389–2393
Hedberg CA (1994) Spermine and spermidine hydroxycinnamoyl transferases in Aphelandra tetragona (Vahl) Nees. Hydroxamic acids, their glucosides, and Benzoxazolinones in the genus Aphelandra (Acanthaceae). In: Inaugural Thesis, Philosophical Faculty II of the University Zürich
Henry M, Roussel JL & Andary C (1987) Verbascoside production in callus and suspension cultures of Hygrophila erecta. Phytochemistry 26: 1961–1963
Herbert JM, Maffrand JP, Taoubi K, Augereau JM, Fouraste I & Gleye J (1991) Verbascoside isolated from Lanata-camara an inhibitor of protein kinase C. J. Nat. Prod. 54: 1595–1600
Inagaki N, Nishimura H, Okada M & Mitsuhashi H (1991) Verbascoside production by plant cell cultures. Plant Cell Rep. 9: 484–487
Kernan MR, Amarquaye A, Chen JL, Chan J, Sesin DF, Parkinson N, Ye ZJ, Barrett M, Bales C, Stoddart CA, Sloan B, Blanc P, Limbach C, Mrisho S & Rozhon EJ (1998) Antiviral phenylpropanoid glycosides from the medicinal plant Markhamia lutea. J. Nat. Prod. 61: 564–570
Ketchum REB, Gibson DM & Gallo LG (1995) Media optimization for maximum biomass production in cell cultures of pacific yew. Plant Cell, Tiss. Org. Cult. 42: 185–193
Kimura Y, Okuda H, Nishibe S & Arichi S (1987) Effects of caffeoylglycosides on arachidonate metabolism in leukocytes. Planta Medica 53: 148–153
Matsumoto M, Koga S, Shoyama Y & Nishioka I (1987) Phenolic glycoside composition of leaves and callus cultures of Digitalis purpurea. Phytochemistry 26: 3225–3227
Mei YH (1994) A sensitive and fast method for the determination of polyamines in biological samples. Benzoyl chloride pre-column derivatization high-performance liquid chromatography. J. Liq. Chromatogr. 17: 2413–2418
Miyase T, Koizumi A, Ueno A, Noro T, Kuroyanagi M, Fukushima S, Akiyama Y & Takemoto T (1982) Studies on the acyl glycosides from Leucoseptrum japonicum (MIQ.) Kitamura et Murata. Chem. Pharm. Bull. 30: 2732–2737
Murashige T & Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15: 473–497
Numata A, Pettit GR, Nabae M, Yamamoto K, Yamamoto E, Matsumura E & Kawano T (1987) Assignment of quaternary carbons in aromatic compounds by long-range heteronuclear shift correlated 2D-NMR spectroscopy and its application to acteoside. Agric. Biol. Chem. 51: 1199–1201
Papazoglou G, Sierre J, Homberger K, Guggisberg A, Woggon WD & Hesse M (1991) Biosynthesis of the spermine alkaloid aphelandrine. Helv. Chim. Acta 74: 565–571
Pletsch M, Piacente S, Pizza C & Charlwood BV (1993) The accumulation of phenylpropanoid glycosides in tissue cultures of Tecoma sambucifolium. Phytochemistry 34: 161–165
Shoyama Y, Matsumoto M & Nishioka I (1986) Four caffeoyl glycosides from callus tissue of Rehmannia glutinosa. Phytochemistry 25: 1633–1636
Slocum RD, Flores HE, Galston AW & Weinstein LH (1989) Improved method for HPLC analysis of polyamines agmatine and aromatic monoamines in plant tissue. Plant Physiology 89: 512–517
Wasshausen DC (1975) The genus Aphelandra (Acanthaceae) Smithonian contributions to Botany 18, Smithonian Institution Press Washington (134 pp).
Werner C, Hedberg C, Lorenzi-Riatsch A & Hesse M (1993)Accumulation and metabolism of the spermine alkaloid aphelandrine in roots of Aphelandra tetragona. Phytochemistry 33: 1033–1036
Werner C, Hu W, Lorenzi-Riatsch A & Hesse M (1995) Dicoumaroylspermidines and tri-coumaroylspermidines in anthers of different species of the genus Aphelandra. Phytochemistry 40: 461–465
Werner C, Petrini O & Hesse M (1997) Degradation of the polyamine alkaloid aphelandrine by endophytic fungi isolated from Aphelandra tetragona. FEMS Microbiology Letters 155: 147–153
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nezbedová, L., Hesse, M., Dušek, J. et al. Chemical potential of Aphelandra sp. cell cultures. Plant Cell, Tissue and Organ Culture 58, 133–140 (1999). https://doi.org/10.1023/A:1006363612428
Issue Date:
DOI: https://doi.org/10.1023/A:1006363612428