Abstract
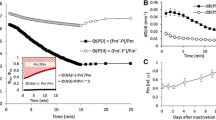
Tobacco plants were subjected to long-term CO2 deficit. The stress caused photoinhibition of Photosystem (PS) II photochemistry and the aggregation of the light-harvesting complex of PS II (LHC II). The aggregation was shown by the appearance of the characteristic band at 698–700 nm (F699) in 77 K fluorescence emission spectra. LHC II aggregates are considered to quench fluorescence and, therefore, the fluorescence yield was determined to verify their quenching capability. PS II photochemistry, measured as FV/FM, was largely depressed during first 4 days of the stress. Unexpectedly, the total fluorescence yield increased in this period. Fitting of emission spectra by Gaussian components approximating emission bands of LHC II, PS II core, PS I and F699 revealed that mainly the bands at 680 and 699 nm, representing emission of LHC II aggregates, were responsible for the increase of the fluorescence yield. This shows an interruption of the excitation energy transfer between LHC II and both photosystems and, thus, a physical disconnection of LHC II from photosystems. PS II and PS I emissions were not quenched in this period. Therefore, it was concluded that these LHC II aggregates were accumulated out of PS II antenna, and, thus they cannot be involved in dumping of excess excitation. The total fluorescence yield turned to decrease only after the large depression of PS II photochemistry, when LHC II aggregation was considerably speeded up and the fluorescence yields of PS I and II turned to decline.
Similar content being viewed by others
References
Bassi R and Dianese P (1992) Supramolecular light-harvesting complex from chloroplast Photosystem II membranes. Eur J Biochem 204: 317–326
Bernard M and Schoefs B (1999) Photosynthetic pigment metabolism in plants during stress. In: Pessarakli M (ed) Handbook of Plant and Crop Stress, pp 527–543. Marcel Dekker, New York/Basel/Hong Kong
Briantais JM, Vernotte C, Picaud M and Krause H (1979) A quantitative study of the slow decline of chlorophyll a fluorescence in isolated chloroplasts. Biochim Biophys Acta 548: 128–138
Burke JJ, Ditto CL and Arntzen CJ (1978) Involvement of the light-harvesting complex in cation regulation of excitation energy distribution in chloroplasts. Arch Biochem Biophys 187: 252–263
Crofts AR and Yerkes CT (1994) A molecular mechanism for QE-quenching. FEBS Lett 352: 265–270
Broyde SB and Brody SS (1966) Spectral studies of a chlorophyll pigment with fluorescence maximum at 698 mµ. Biophys J 6: 353–366
Demming-Adams B (1990) Carotenoids and photoprotection: A role for the xanthophyll zeaxanthin. Biochim Biophys Acta 1020: 1–24
Garab G, Kieleczava J, Sutherland JC, Bustamante C and Hind C (1991) Organisation of pigment–protein complexes into macrodomains in the thylakoid membranes of wild-type and chlorophyll b-less mutant of burley as revealed by circular dichroism. Photochem Photobiol 54: 273–281
Gilmore AM and Yamamoto HY (1992) Dark induction of zeaxanthin-dependent nonphotochemical fluorescence quenching mediated by ATP. Proc Natl Acad Sci USA 89: 1899–1903
Gilmore AM and Yamamoto HY (1993) Linear models relating xanthophylls and lumen acidity to non-photochemical fluorescence quenching. Evidence that antheraxanthin explains zeaxanthin-independent quenching. Photosynth Res 35: 67–78
Hayden DB, Baker NR, Percival MP and Beckwith PB (1986) Modification of the Photosystem II light-harvesting chlorophyll a/b-protein complex in maize during chill-induced photoinhibition. Biochim Biophys Acta 851: 86–92
Hogan GD (1992) Physiological effects of direct impact of acidic deposition on foliage. Agriculture, Ecosystems and Environ 42: 307–319
Heber U, Neimanis S, Siebke K, Schönknecht G and Katona E (1992) Chloroplast energization and oxidation of P700/plastocyanin in illuminated leaves at reduced levels of CO2 or oxygen. Photosynth Res 34: 433–447
Horton P and Ruban AV (1992) Regulation of Photosystem II. Photosynth Res 34: 375–385
Horton P, Ruban AV, Rees D, Pascal AA, Noctor G and Young AJ (1991) Control of the light-harvesting function of chloroplast membranes by aggregation of the LHC II chlorophyll–protein complex. FEBS Lett 242: 1–4
Horton P, Ruban AV and Walters RG (1994) Regulation of light harvesting in green plants. Identification by nonphotochemical quenching of chlorophyll fluorescence. Plant Physiol 106: 415–420
Horton P, Ruban AV and Walters RG (1996) Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol 47: 665–684
Ikegami I (1976) Fluorescence changes related in the primary photochemical reactions in the P-700-enriched particles isolated from spinach chloroplasts. Biochim. Biophys Acta 449: 245–258
Ikegami I and Ke B (1984) A 160-kilodalton Photosystem I reaction-center complex. Low-temperature fluorescence spectroscopy. Biochim Biophys Acta 764: 80–85
Jennings RC, Garlaschi FM and Zucchelli G (1991) Light-induced fluorescence quenching in the light-harvesting chlorophyll a/b-protein complex. Photosynth Res 27: 57–64
Kolubayev T, Geacintov NE, Paillotin G and Breton J (1986) Domain size in chloroplasts and chlorophyll–protein complexes probed by fluorescence yield quenching induced by singlet-triplet exciton annihilation. Biochim Biophys Acta 376: 105–115
Lebedev NN, Khatyrov RA, Ladygin VA and Krasnovskii AA (1988) Fluorescence excitation spectra and decay kinetics of light-harvesting complex in Chlamydomonas reinhardtii mutants. Photosynthetica 22: 364–370
Lichtenthaler HK (1987) Chlorophylls and carotenoids: Pigments of photosynthetic membranes. In: Packer L and Douce R (eds) Methods in Enzymology, Vol 148, pp 350–382. Academic Press, New York/San Francisco, London
Mullet JE and Arntzen CJ (1980) Simulation of grana stacking in a model system. Mediation by a purified light-harvesting pigment-protein complex from chloroplasts. Biochim Biophys Acta 589: 100–117
Mullineaux CW, Ruban AV and Horton P (1994) Prompt heat release associated with ΔpH-dependent quenching in spinach thylakoid membranes. Biochim Biophys Acta 1185: 119–123
Murata N and Satoh K (1986) Absorption and fluorescence emission by intact cells, chloroplasts and chlorophyll–protein complexes. In: Govindjee, Amez J and Fork DC (eds) Light Emission by Plants and Bacteria, pp 137–159. Academic Press, London
Noctor G, Ruban AV and Horton P (1993) Modulation of ΔpH-dependent nonphotochemical quenching of chlorophyll fluorescence in spinach chloroplasts. Biochim Biophys Acta 1183: 336–344
Oxborough K and Horton P (1987) Characterization of the effect of Antimycin A upon high energy state quenching of chlorophyll fluorescence (qE) in spinach and pea chloroplasts. Photosynth Res 12: 119–128
Peter GF and Thornber JP (1991) Biochemical composition and organization of higher plant Photosystem II light harvesting pigment proteins. J Biol Chem 266: 16745–16754
Phillip D, Ruban AV, Horton P, Asato Al and Young AJ (1996) Quenching of chlorophyll fluorescence in the major light-harvesting complex of Photosystem II: A systematic study of the effect of carotenoid structure. Proc Natl Acad Sci USA 93: 1492–1497
Ruban AV and Horton P (1992) Mechanism of ΔpH-dependent dissipation of absorbed excitation energy by photosynthetic membranes I. Spectroscopic analysis of isolated light-harvesting complexes. Biochim Biophys Acta 1102: 30–38
Ruban AV and Horton P (1994) Spectroscopy of non-photochemical and photochemical quenching of chlorophyll fluorescence in leaves; evidence for a role of the light harvesting complex of Photosystem II in regulation of energy dissipation. Photosynth Res 40: 181–190
Ruban AV and Horton P (1995) Regulation of non-photochemical quenching of chlorophyll fluorescence in plants. Aust J Plant Physiol 22: 221–230
Ruban AV, Rees D, Noctor GD, Young A and Horton P (1991) Long-wavelength chlorophyll species are associated with amplification of high-energy-state excitation quenching in higher plants. Biochim Biophys Acta 1059: 355–360
Ruban AV, Rees D, Pascal AA and Horton P (1992a) Mechanism of pH-dependent dissipation of absorbed excitation energy by photosynthetic membranes II. The relationship between LHC II aggregation in vitro and qE in isolated thylakoids. Biochim Biophys Acta 1102: 39–44
Ruban AV, Walters RG and Horton P (1992b) The molecular mechanism of the control of excitation energy dissipation in chloroplast membranes: inhibition of ΔpH-dependent quenching of chlorophyll fluorescence by dicyclohexylcarbodiimide. FEBS Lett 309: 175–179
Ruban AV, Young AJ and Horton P (1993) Induction of nonphotochemical energy dissipation and absorbance changes in leaves. Plant Physiol 102: 741–750
Ruban AV, Young AJ and Horton P (1994) Modulation of chlorophyll fluorescence quenching in isolated light harvesting complex of Photosystem II. Biochim Biophys Acta 1186: 123–127
Ruban AV, Young AJ and Horton P (1996) Dynamic properties of the minor chlorophyll a/b binding proteins of Photosystem II, an in vitro model for photoprotective energy dissipation in the photosynthetic membrane of green plants. Biochemistry 35: 674–678
Ruban AV, Phillip D, Young AJ and Horton P (1997) Carotenoid-dependent oligomerization of the major chlorophyll a/b light harvesting complex of Photosystem II of plants. Biochemistry 36: 7855–7859
Ryrie IJ, Anderson JM and Goodchild DJ (1980) The role of the light-harvesting chlorophyll a/b–protein complex in chloroplast membrane stacking. Cation induced aggregation of reconstituted proteoliposomes. Eur J Biochem 107: 345–354
Schreiber U and Armond PA (1978) Heat-induced changes of chlorophyll fluorescence in isolated chloroplasts and related heat-damage at the pigment level. Biochim Biophys Acta 502: 138–151
Š P, Braunova Z, Šindelkova E and Cudlín P (1996) The effect of simulated acid rain on chlorophyll fluorescence spectra of spruce seedlings (Picea abies L. Karst). J Plant Physiol 148: 271–275
Š P, Kutík M and Lebedev NN (1991) Spectroscopically analyzed degradation of chlorophyll–protein complexes and chloroplasts ultrastructure during yellowing of leaves. Photosynthetica 25: 395–407
Š P and Vácha F (1998) Aggregation of the light-harvesting complex in intact leaves of Tobacco plants stressed by CO2 deficit. Photochem Photobiol 67: 304–311
Stys D, Stancek M, Chen L and Allen JF (1995) Complex formation in plant thylakoid membrane. Competition studies on membrane protein interactions using synthetic peptide fragments. Photosynth Res 44: 227–285
Yamane Y, Kashino Y, Koike H and Satoh K (1997) Increase in the fluorescence F0 level and reversible inhibition of Photosystem II reaction center by high-temperature treatment in higher plants. Photosynth Res 52: 57–64
Rights and permissions
About this article
Cite this article
Siffel, P., Braunová, Z. Release and aggregation of the light-harvesting complex in intact leaves subjected to strong CO2 deficit. Photosynthesis Research 61, 217–226 (1999). https://doi.org/10.1023/A:1006298111894
Issue Date:
DOI: https://doi.org/10.1023/A:1006298111894