Abstract
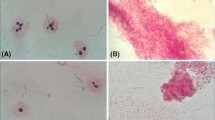
Pigment producing in vitro cells of Vaccinium pahalae (ohelo) were tested for their ability to survive cryopreservation and retain pigment-production capacity after encapsulation-dehydration. Preculture of cells for 6 to 8 days in a medium containing 1.0 M sucrose was essential before dehydration. Reduction of bead water content before quenching in liquid nitrogen was highly correlated (r = 0.94) with increased survival rate in cells after cryopreservation. Dehydration of beads for 4 h was satisfactory for survival of cells. After quenching in liquid nitrogen, colored cells became pale, but pigment content was recovered once cells resumed growth. After three subcultures, cells regained their maximum capacity for pigment accumulation. The percentage of colored-to-total cell volume was not influenced by cryopreservation. Encapsulation-dehydration and cryopreservation did not diminish the capacity of cells to produce anthocyanins and other flavonoids.
Similar content being viewed by others
References
Bachiri Y, Gazeau C, Hansz J, Morisset C & Dereuddre J (1995) Successful cryopreservation of suspension cells by encapsulation-dehydration. Plant Cell Tiss. Org. Cult. 43: 241–248
Chen HHT, Kartha KK, Leung NL, Kurz WGW, Chaston KB & Constable F (1984) Cryopreservation of alkaloid producing cell cultures of periwinkle (Catharanthus roseus). Plant Physiol. 75: 726–731
Dereuddre J, Blandin S & Hassen N (1991) Resistance of alginate-coated somatic embryos of carrot (Daucus carota L.) to desiccation and freezing in liquid nitrogen: I. Effect of preculture. Cryo-Lett. 12: 125–134
Hatanaka T, Yasuda T, Yamaguchi T & Sakai A (1994) Direct regrowth of encapsulated somatic embryos of coffee (Coffea canephora) after cooling in liquid nitrogen. Cryo-Lett. 15: 47–52
Lloyd G & McCown B (1980) Commercially-feasible micropropagation of mountain laurel Kalmia latifolia, by use of shoot tip culture. Proc. Intl. Plant Prop. Soc. 30: 421–427
Madhavi DL, Smith MAL & Rogers R (1995) Expression of anthocyanins in bilberry and huckleberry callus cultures. In Vitro Cell. Devel. Biol. 31: 66A. Abstr.# P-1072
Mannonen L, Toivonen L & Kauppinen V (1990) Effect of long term preservation on growth and productivity of Panax ginseng and Catharanthus roseus cell suspensions. Plant Cell Rep. 9: 173–177
Moster JB & Quackenbush FV (1952) The effects of temperature and light on the carotenoids of seedlings grown from three corn hybrids. Arch. Bioch. Biophysic. 38: 297–303
Niino T & Sakai A (1992) Cryopreservation of alginate-coated in vitro grown shoot tips of apple, pear, and mulberry. Plant Sci. 87: 199–206
Paulet F, Engelmann F & Glaszmann J-C (1993) Cryopreservation of apices of in vitro plantlets of sugarcane (Saccahrum sp. hybrids) using encapsulation/dehydration. Plant Cell Rep. 12: 525–529
Reinhoud PJ, Schrijnemakers EWM, Van Iren F & Kijine JW (1995) Vitrification and a heat-shock treatment improve cryopreservation of tobacco cell suspension compared to two-step freezing. Plant Cell Tiss. Org. Cult. 42: 261–267
Sato K, Nakayama M & Sheigeta J (1996) Culturing conditions affecting the production of anthocyanin in suspended cell cultures of strawberry. Plant Science 113: 91–98
Schrijnemakers EWM & Van Iren F (1995) A two-step or equilibrium freezing procedure for the cryopreservation of plant cell suspensions. In: Day JG & MR McLellan (eds) Methods in Molecular Biology. Vol. 38. Cryopreservation and Freeze-drying Protocols. (pp 418–421). Humana Press Inc., Totowa, NJ
Scottez C, Chevreau E, Godard N, Arnaud Y, Duron M & Dereuddre J (1992) Cryopreservation of cold-hardened shoot tips of pear (Pyrus communis L. cv. Beurre Hardy) in vitro cultures after encapsulated-dehydration. Cryobiology 29: 691–700
Shibli RA & Smith MAL (1996) Direct shoot regeneration from Vaccinium pahalae (Ohelo) and V. myrtillus (Bilberry) leaf explants. HortScience 31: 1225–1228
Shibli RA, Smith MAL & Kushad M (1997) Headspace ethylene effects secondary metabolites in Vaccinium pahalae cell culture. Pl. Growth. Reg. 23: 201–205
Smith MAL, Madhavi DL, Fang Y & Tomczak MM (1997) Continuous cell culture and product recovery from wild Vaccinium pahalae germplasm. J. Plant Physiol. 150: 462–466
Steponkus PL & Lanphear FO (1967) Refinement of the triphenyl tetrazolium chloride method of determining cold injury. Plant Physiol. 42: 1423–1426
Towill LE (1988) Genetic considerations for germplasm preservation of clonal materials. HortScience. 23: 91–95
Towill LE (1996a) Germplasm preservation. In: Trigiano RN & DJ Gray (eds). Plant Tissue Culture Concepts and Laboratory Exercises (pp 291–296). CRC Press. Boca Raton
Towill LE (1996b) Vitrification as a method to cryopreserved shoot tips. In: Trigiano RN & DJ Gray (eds). Plant Tissue Culture Concepts and Laboratory Exercises (pp 297–304). CRC Press. Boca Raton, New York, London, Tokyo
Uragami A, Sakai A & Nagai M (1990) Cryopreservation of dried axillary buds from plantlets of Asparagus officinalis L. grown in vitro. Plant Cell Rep. 9: 328–331
Uragami A, Sakai A, Nagai M & Takahashi T (1989) Survival of cultured cells and somatic embryos of Asparagus officinalis cryopreserved by vitrification. Plant Cell Rep. 8: 418–421
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shibli, R.A., Smith, M. & Shatnawi, M. Pigment recovery from encapsulated-dehydrated Vaccinium pahalae (ohelo) cryopreserved cells. Plant Cell, Tissue and Organ Culture 55, 119–123 (1998). https://doi.org/10.1023/A:1006276205723
Issue Date:
DOI: https://doi.org/10.1023/A:1006276205723