Abstract
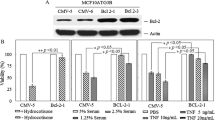
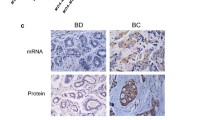
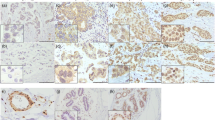
Basic fibroblast growth factor (bFGF, FGF–2), a classical transforming factor, mitogen, and survival factor in multiple cell types, and has a paradoxic role in mammary epithelial cell transformation and proliferation. We have also demonstrated that recombinant FGF–2 uncharacteristically promotes cell death in MCF–7 human breast cancer cells. In this study, we investigated the effects of FGF–2 overexpression on survival in the same MCF–7 cells. In eight breast cancer cell lines and two nontransformed mammary epithelial cell lines, we demonstrated that high levels of Bcl–2 are only expressed in cells with undetectable levels of FGF–2 on western blot. In retrovirally transduced MCF–7 cells expressing both cytoplasm– and nucleus–localizing FGF–2 species and ones expressing only cytoplasm–localizing FGF–2 species, Bcl–2 levels were strongly decreased at both the mRNA and protein levels. Immunoprecipitation of Bax demonstrated a decreased association of Bax with Bcl–2 in these cells. Levels of Bax did not correlate with expression of FGF–2 in the 10 cell lines or in MCF–7 cells. The clonogenic potential of MCF–7 cells in tissue culture was decreased by the expression of FGF–2 and was additively suppressed by the chemotherapeutic agents etoposide and 5–fluorouracil in a dose and time dependent manner. MCF–7 cells overexpressing FGF–2 had a greater rate of programmed cell death at baseline and in response to etoposide and 5–fluorouracil in a TUNEL assay by immunofluorescent microphotography and by flow cytometric quantitation. The pro–apoptotic effect of FGF–2 overexpression on the chemosensitivity of these cells was confirmed by quantitative morphologic determination. These data demonstrate that the expression of FGF–2 downregulates Bcl–2 and promotes programmed cell death in MCF–7 human breast cancer cells.
Similar content being viewed by others
References
Burgess WH, Maciag T: The heparin-binding (fibroblast) growth factor family of proteins. Ann Rev Biochem 58: 575–606, 1989
Folkman J: The role of angiogenesis in tumor growth. Semin Cancer Biol 3: 65–71, 1992
Florkiewicz RZ, Baird A, Gonzalez, AM: Multiple forms of bFGF: differential nuclear and cell surface localization. Growth Factors 4: 265–275, 1991
Maciag T, Zhan X, Garfinkel S, Friedman S, Prudovsky I, Jackson A, Wessendorf J, Hu X, Gamble S, Shi J, Brown S, Tarantini F, Zimrin A: Novel mechanisms of fibroblast growth factor 1 function. Rec Progress Hormone Res 49: 105–122, 1994
Luqmani YA, Graham M, Coombes RC: Expression of basic fibroblast growth factor, FGFR1 and FGFR2 in normal and malignant human breast, and comparison with other normal tissues. Br J Cancer 66: 273–280, 1992
Anandappa SY, Winstanley JHR, Leinster S, Green B, Rudland PS, Barraclough R: Comparative expression of fibroblast growth factor mRNAs in benign and malignant breast disease. Br J Cancer 69: 772–776, 1994
Gomm JJ, Smith J, Ryall GK, Baille R, Turnbull L, Coombes RC: Localization of basic fibroblast growth factor and transforming growth factor b1 in the human mammary gland. Cancer Res 51: 4685–4692, 1991
Yiangou C, Gomm JJ, Coope RC, Law M, Luqmani YA, Shousha S, Coombes RC, Johnston CL: Fibroblast growth factor 2 in breast cancer: occurrence and prognostic significance. Br J Cancer 75: 28–33, 1997
Colomer R, Aparicio J, Montero S, Guzman C, Larrodera L, Cortes-Funes H: Low levels of basic fibroblast growth factor (bFGF) are associated with a poor prognosis in human breast carcinoma. Br J Cancer 76: 1215–1220, 1997
McLeskey SW, Ding IYF, Lipman ME, Kern FG: MDAMB-134 breast carcinoma cells overexpress fibroblast growth factor (FGF) receptors and are growth-inhibited by FGF ligands. Cancer Res 54: 523–530, 1994
Fenig E, Wieder R, Paglin S, Wang H, Persaud R, Haimovitz-Friedman A, Fuks Z, Yahalom J: Basic fibroblast growth factor confers growth inhibition and mitogen-activated protein kinase activation in human breast cancer cells. Clin Cancer Res 3: 135–142, 1997
Wang H, Rubin M, Fenig E, DeBlasio T, Mendelsohn J, Yahalom J, Wieder R: Basic FGF causes growth arrest in MCF-7 human breast cancer cells while inducing both mitogenic and inhibitory G1 events. Cancer Res 57: 1750–1757, 1997
Wieder R, Fenig E, Wang H, Paglin S, Wang Q, Menzel T, Gabrilove J, Fuks Z, Yahalom J: Overexpression of basic fibroblast growth factor in MCF-7 human breast cancer cells: lack of correlation between inhibition of cell growth and MAP kinase activation. J Cell Phys 177: 411–425, 1998
Fuks Z, Persaud RS, Alfieri A, McLoughlin M, Ehleiter D, Schwartz JL, Seddon AP, Cordon-Cardo C, Haimovitz-Friedman A: Basic fibroblast growth factor protects endothelial cells against radiation-induced programmed cell death in vitro and in vivo. Cancer Res 54: 2582–2590, 1994
Haimovitz-Friedman A, Balaban N, McLoughlin M, Ehleiter D, Michaeli J, Vlodavsky I, Fuks Z: Protein kinase C mediates basic fibroblast growth factor protection of endothelial cells against radiation-induced apoptosis. Cancer Res 54: 2591–2597, 1994
Wieder R, Wang H, Shirke S, Wang Q, Menzel T, Feirt N, Jakubowski AA, Gabrilove JL: Low level expression of basic FGF upregulates Bcl-2 and delays apoptosis, but high intracellular levels are required to induce transformation in NIH 3T3 cells. Growth Factors 15: 41–60, 1997
Konig A, Menzel T, Lynen S, Wrazel L, Rosen A, Al-Katib A, Raveche E, Gabrilove JL: Basic fibroblast growth factor (bFGF) upregulates the expression of bcl-2 in B cell chronic lymphocytic leukemia cell lines resulting in delaying apoptosis. Leukemia 11: 258–265, 1997
Menzel T, Rahman Z, Calleja E, White K, Wilson EL, Wieder R, Gabrilove J: Elevated intracellular level of basic fibroblast growth factor correlates with stage of chronic lymphocytic leukemia and is associated with resistance to fludarabine. Blood 87: 1056–1063, 1996
Wang Q, Maloof P, Wang H, Fenig E, Stein D, Nichols G, Denny TN, Yahalom J, Wieder R: Basic fibroblast growth factor (bFGF) downregulates Bcl-2 and promotes apoptosis in MCF-7 human breast cancer cells. Exp Cell Res 238: 177–187, 1998
Hacker G, Vaux DL: A sticky business. Current Biol 5: 622–624, 1995
Yin XM, Oltvai ZN, Korsmeyer SJ: BH1 and BH2 domains of bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature 369: 321–323, 1994.
Soule HD, Maloney TM, Wolman SR, Peterson WDJr, Brenz R, McGrath CM, Russo J, Pauley RJ, Jones RF, Brooks SC: Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res 50: 6075–6086, 1990
Konopka JB, Witte ON: Detection of c-abl tyrosine kinase activity in vitro permits direct comparison of normal and altered abl gene products. Mol Cell Biol 5: 3116–3123, 1985
Chomczynski P, Sacehi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987
Alwine JC, Kemp DJ, Stark GR: Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Nat Acad Sci USA 74: 5350–5354, 1997
Hotz MA, Gong J, Traganos F, Darzynkiewicz Z: Flow cytometric detection of apoptosis: comparison of the assays of in situ DNA degradation and chromatin changes. Cytometry 15: 237–244, 1994
Fox J, Shanley J: Antisense inhibition of basic fibroblast growth factor induces apoptosis in vascular smooth muscle cells. J Biol Chem 271: 12578–12584, 1996
Chow RL, Roux GD, Roghani M, Palmer MA, Rifkin DB, Moscatelli DA, Lang RA: FGF suppresses apoptosis and induces differentiation of fibre cells in the mouse lens. Development 121: 4383–4393, 1995
Yasuda T, Grinspan J, Stern J, Francesehini B, Bannerman P, Pleasure D: Apoptosis occurs in the oligodendroglial lineage, and is prevented by basic fibroblast growth factor. J Neurosci Res 40: 306–317, 1995
Miyake H, Hara I, Gohji K, Yoshimura K, Arakawa S: Expression of basic fibroblast growth factor is associated with resistance to cisplatin in a human bladder cancer cell line. Cancer Lett 123: 121–126, 1998
Karsan A, Yee E, Poirier GG, Zhou P, Craig R, Harlan JM: Fibroblast growth factor-2 inhibits endothelial cells apoptosis by Bcl-2-dependent and independent mechanisms. Am J Pathol 151: 1775–1784, 1997
Trolice MP, Pappalardo A, Pelusso JJ: Basic fibroblast growth factor and N-cadherin maintain rat granulosa cell and ovarian surface epithelial cell viability by stimulating the tyrosine phosphorylation of the fibroblast growth factor receptors. Endocrinology 138: 107–113, 1997
Gardner AM, Johnson GL: Fibroblast growth factor-2 suppression of tumor necrosis factor a-mediated apoptosis requires Ras and the activation of mitogen-activated protein kinase. J Biol Chem 271: 14560–14566, 1996
Seto M, Jaeger U, Hockett RD, Graninger W, Bennett S, Goldman P, Korsmeyer SJ: Alternative promoters and exons, somatic mutation and deregulation of the Bcl-2-1g fusion gene in lymphoma. EMBO J 7: 123–131, 1998
Young RL, Korsmeyer SJ: A Negative Regulatory Element in the bcl-2 50-untranslated region inhibits expression from an upstream promoter. Mol Cell Biol 13: 3686–3697, 1993
Rowe M, Peng-Pilon M, Huen DS, Hardy R, Croom-Carter D, Lundgren E, Rickinson AB: Upregulation of bcl-2 by the Epstein-Barr virus latent membrane protein LMP1: a B-cellspecific response that is delayed relative to NF-кB activation and to induction of cell surface markers. J Virol 68: 5602–5612, 1994
Barbaresehi M, Caffo O, Veronese S, Leek RD, Fina P, Fox S, Bonzanini M, Girlando S, Morelli L, Eccher C, Pezzella F, Doglioni C, Dalla Palma P, Harris A: Bcl-2 and p53 expression in node-negative breast carcinoma: a study with long-term follow-up. Human Pathol 27: 1149–1155, 1996
Steck K, McDonnell T, Sneige N, El-Naggar A: Flow cytometric analysis and bcl-2 primary breast carcinomas: clinical and biological implications. Cytometry 24: 116–122, 1996
Bukholm IK, Nesland JM, Karesen R, Jacobsen U, Borresen-Dale AL: Interactions between bcl-2 and p21 (WAF1/CIP1) in breast carcinomas with wild-type p53. Int J Cancer 73: 38–41, 1997
Charpin C, Garcia S, Bouvier C, Devictor B, Andrac L, Lavaut MN, Allasia C: Automated and quantitative immunocytochemical assays of Bcl-2 protein in breast carcinomas. Br J Cancer 76: 340–346, 1997
Elledge RM, Green S, Howes L, Clark GM, Berardo M, Allred DC, Pugh R, Ciocca D, Ravdin P, O'Sullivan J, Rivkin S, Martino S, Osborne CK: Bcl-2, p53, and response to tamoxifen in estrogen receptor-positive metastatic breast cancer: a Southwest Oncology Group study. J Clin Oncol 15: 1916–1922, 1997
Hori M, Nogami T, Itabashi M, Yoshimi F, Ono H, Koizumi S: Expression of Bcl-2 in human breast cancer: correlation between hormone receptor status, p53 protein accumulation and DNA strand breaks associated with apoptosis. Pathol Int 47: 757–762, 1997
Kobayashi S, Iwase H, Ito Y, Yamashita H, Iwata H, Yamashita T, Ito K, Toyama T, Nakamura T, Masaoka A: Clinical signi-ficance of bcl-2 gene expression in human breast cancer tissue. Breast Cancer Res Treat 42: 173–181, 1997
Berardo MD, Elledge RM, de Moor C, Clark GM, Osborne CK, Allred DC: Bcl-2 and apoptosis in lymph node positive carcinoma. Cancer 82: 1296–1302, 1998
Teixeira C, Reed JC, Pratt MA: Estrogen promotes chemotherapeutic drug resistance by a mechanism involving Bcl-2 proto-oncogene expression in human breast cancer cells. Cancer Res 55: 3902–3907, 1995
Wang TT, Phang JM: Effects of estrogen on apoptotic pathways in human breast cancer cell line MCF-7. Cancer Res 55: 2487–2489, 1995
Huang Y, Ray S, Reed JC, Ibrado AM, Tang C, Nawabi A, Bhalla K: Estrogen increases intracellular p26Bcl-2 to p2lBax ratios and inhibits taxol-induced apoptosis of human breast cancer MCF-7 cells. Breast Cancer Res Treat 42: 73–81, 1997
Zapata JM, Krajewska M, Krajewski S, Huang RP, Takayama S, Wang HG, Adamson E, Reed JC: Expression of multiple apoptosis-regulatory gene in human breast cancer cell lines and primary tumors. Breast Cancer Res Treat 47: 129–140, 1998
Kapranos N, Karaiosifidi H, Valavanis C, Kouri E, Vasilaros S: Prognostic significance of apoptosis related proteins Bcl-2 and Bax in node-negative breast cancer patients. Anticancer Res 17: 2499–2505, 1997
van Slooten HJ, van de Vijver MJ, van de Velde CJ, van Dierendonek JH: Loss of Bcl-2 in invasive breast cancer is associated with high rates of cell death, but also with increased proliferative activity. Br J Cancer 77: 789–796, 1998
Sierra A, Lloveras B, Castellsague X, Moreno L, Garcia-Ramirez M, Fabra A: Bcl-2 expression is associated with lymph node metastasis in human ductal breast carcinoma. Int J Cancer 60: 54–60, 1995
Mustonen M, Raunio H, Paakko P, Soini Y: The extent of apoptosis is inversely associated with bcl-2 expression in premalignant and malignant breast lesions. Histopathology 31: 347–354, 1997
Lai LC, Sirah AK, Erbas H, Lennard TWJ: Relationship between basic fibroblast growth factor and transforming growth factor-beta 2 in breast cyst fluid. J Clin Endo Metab 80: 711–714, 1995
Relf M, LeJeune S, Scott PA, Fox S, Smith K, Leek R, Moghaddam A, Whitehouse R, Bicknell R, Harris AL: Expression of the angiogenic factors vascular endothelial cell growth factor, acidic and basic fibroblast growth factor, tumor growth factor beta-1, platelet-derived endothelial cell growth factor, placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res 57: 963–969, 1997
Yoshiji H, Harris SR, Thorgeirsson UP: Vascular endothelial growth factor is essential for initial but not continued in vivo growth of human breast carcinoma cells. Cancer Res 57: 3924–3928, 1997
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Maloof, P., Wang, Q., Wang, H. et al. Overexpression of basic fibroblast growth factor (FGF–2) downregulates Bcl–2 and promotes apoptosis in MCF–7 human breast cancer cells. Breast Cancer Res Treat 56, 151–165 (1999). https://doi.org/10.1023/A:1006258510381
Issue Date:
DOI: https://doi.org/10.1023/A:1006258510381