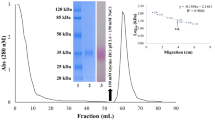
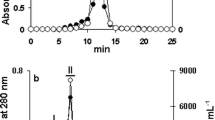
Abstract
Urtica dioica agglutinin (UDA) has previously been found in roots and rhizomes of stinging nettles as a mixture of UDA- isolectins. Protein and cDNA sequencing have shown that mature UDA is composed of two hevein domains and is processed from a precursor protein. The precursor contains a signal peptide, two in-tandem hevein domains, a hinge region and a carboxyl-terminal chitinase domain. Genomic fragments encoding precursors for UDA-isolectins have been amplified by five independent polymerase chain reactions on genomic DNA from stinging nettle ecotype Weerselo. One amplified gene was completely sequenced. As compared to the published cDNA sequence, the genomic sequence contains, besides two basepair substitutions, two introns located at the same positions as in other plant chitinases. By partial sequence analysis of 40 amplified genes, 16 different genes were identified which encode seven putative UDA- isolectins. The deduced amino acid sequences share 78.9–98.9% identity. In extracts of roots and rhizomes of stinging nettle ecotype Weerselo six out of these seven isolectins were detected by mass spectrometry. One of them is an acidic form, which has not been identified before. Our results demonstrate that UDA is encoded by a large gene family.
Similar content being viewed by others
References
Allen AK, Neuberger A, Sharon N: The purification, composition and specificity of WGA. Biochem J 131: 155–162 (1973).
Andersen NH, Cao B, Rodríguez-Romero A, Arreguin B: Hevein: NMR assignment and assessment of solution-state folding for the agglutinin-toxin motif. Biochemistry 32: 1407–1422 (1993).
Archer BL: The proteins of Hevea brasiliensis latex. Isolation and characterization of crystalline hevein. Biochem J 75: 236–240 (1960).
Asensio JL, Cañada FJ, Bruix M, Rodriguez-Romero A, Jiminez-Barbero J: The interaction of hevein with N-acetylglucosamine-containing oligosaccharides. Solution structure of hevein complexed to chitobiose. Eur J Biochem 230: 621–633 (1995).
Balzarini J, Neyts J, Schols D, Hosoya M, Van Damme E, Peumans W, De Clercq E: The mannose-specific plant lectins from Cymbidium hybrid and Epipactis helleborine and the (N-acetylglucosamine)n-specific plant lectin from Urtica dioica are potent and selective inhibitors of human immunodeficiency virus and cytomegalovirus replication in vitro. Antiviral Res 18: 191–207 (1992).
Bassett IJ, Crompton CW, Woodland DW: The family Urticaceae in Canada. Can J Bot 52: 503–516 (1974).
Beintema JJ: Structural features of plant chitinases and chitinbinding proteins. FEBS Lett 350: 159–163 (1994).
Beintema JJ, Peumans WJ: The primary structure of stinging nettle (Urtica dioica) agglutinin. A two-domain member of the hevein family. FEBS Lett 299: 131–134 (1992).
Broekaert WF, Mariën W, Terras FRG, De Bolle MFC, Proost P, Van Damme J, Dillen L, Claeys M, Rees SB, Vanderleyden J, Cammue BPA: Antimicrobial peptides from Amaranthus caudatus seeds with sequence homology to the cysteine/glycine-rich domain of chitin-binding proteins. Biochemistry 31: 4308–4314 (1992).
Broekaert WF, Van Parijs J, Leyns F, Joos H, Peumans WJ: A chitin-binding lectin from stinging nettle rhizomes with antifungal properties. Science 245: 1100–1102 (1989).
Chapot MP, Peumans WJ, Strosberg AD: Extensive homologies between lectins from non-leguminous plants. FEBS Lett 195: 231–234 (1986).
Chrispeels MJ, Raikhel NV: Lectins, lectin genes, and their role in plant defense. Plant Cell 3: 1–9 (1991).
Cole RA: Isolation of a chitin-binding lectin, with insecticidal activity in chemically-defined sythetic diets, from two wild brassica species with resistance to cabbage aphid Brevicoryne brassicae. Entomol Exp Appl 72: 181–187 (1994).
Drenth J, Low BW, Richardson JS, Wright CS: The toxinagglutinin fold. A new group of small protein structures organized around a four-disulfide core.J Biol Chem 255: 2652–2655 (1980).
Galelli A, Truffa-Bachi P: Urtica dioica agglutinin. A superantigenic lectin from stinging nettle rhizome. J Immunol 151: 1821–1831 (1993).
Hom K, Gochin M, Peumans WJ, Shine N: Ligand-induced perturbations in Urtica dioica agglutinin. FEBS Lett 361: 157–161 (1995).
Huesing JE, Murdock LL, Shade RE: Rice and stinging nettle lectins: insecticidal activity similar to wheat germ agglutinin. Phytochemistry 30: 3565–3568 (1991).
Lerner DR, Raikhel NV: The gene for stinging nettle lectin (Urtica dioica agglutinin) encodes both a lectin and a chitinase. J Biol Chem 267: 11085–11091 (1992).
Martins JC, Maes D, Loris R, Pepermans HAM, Wyns L, Willem R, Verheyden P: 1H NMR study of the solution structure of Ac-AMP2, a sugar binding antimicrobial protein isolated from Amaranthus caudatus. J Mol Biol 258: 322–333 (1996).
Melchers LS, Sela-Buurlage MB, Vloemans SA, Woloshuk CP, van Roekel JSC, Pen J, van den Elzen PJM, Cornelissen BJC: Extracellular targeting of the vacuolar tobacco proteins AP24, chitinase and β-1,3-glucanase in transgenic plants. Plant Mol Biol 21: 583–593 (1993).
Neuhaus J-M, Fritig B, Linthorst HJM, Meins F Jr, Mikkelsen JD, Ryals J: A revised nomenclature for chitinase genes. Plant Mol Biol Rep 14: 102–104 (1996).
Peumans WJ, De Ley M, Broekaert WF: An unusual lectin from stinging nettle (Urtica dioica) rhizomes. FEBS Lett 177: 99–103 (1984).
Peumans WJ, Stinissen HM, Carlier AR: A genetic basis for the origin of six different isolectins in hexaploid wheat. Planta 154: 562–567 (1982).
Peumans WJ, Van Damme EJM: The role of lectins in plant defence. Histochem J 27: 253–271 (1995).
Peumans WJ, Van Damme EJM: Lectins as plant defense proteins. Plant Physiol 109: 347–352 (1995).
Raikhel NV, Wilkins TA: Isolation and characterization of a cDNA clone encoding wheat germ agglutinin. Proc Natl Acad Sci USA 84: 6745–6749 (1987).
Rice RH: Wheat germ agglutinin. Evidence for a genetic basis of multiple forms. Biochim Biophys Acta 444: 175–180 (1976).
Sambrook J, Fritsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY (1989).
Sanger F, Nicklen S, Coulson AR: DNA sequencing with chain-termination inhibitors. Proc Natl Acad Sci USA 74: 5463–5467 (1977).
Schägger H, Von Jagow G: Tricine-sodium dodecyl sulfatepolyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 166: 368–379 (1987).
Shibuya N, Goldstein IJ, Shafer JA, Peumans WJ, Broekaert WF: Carbohydrate binding properties of the stinging nettle (Urtica dioica) rhizome lectin. Arch Biochem Biophys 249: 215–224 (1986).
Siebert H-C, von der Lieth C-W, Kaptein R, Beintema JJ, Dijkstra K, van Nuland N, Soedjanaatmadja UMS, Rice A, Vliegenthart JFG, Wright CS, Gabius H-J: Role of aromatic amino acids in carbohydrate binding of plant lectins: laser photo chemically induced dynamic nuclear polarization study of hevein domain-containing lectins. Proteins Struct Funct Genet 28: 268–284 (1997).
Smith JJ, Raikhel NV: Nucleotide sequences of cDNA clones encoding wheat germ agglutinin isolectins A and D. Plant Mol Biol 13: 601–603 (1989).
Van Damme EJM, Broekaert WF, Peumans WJ:. The Urtica dioica agglutinin is a complex mixture of isolectins. Plant Physiol 86: 598–601 (1988).
Van Damme EJM, Peumans WJ: Isolectin composition of individual clones of Urtica dioica: evidence for phenotypic differences. Physiol Plant 71: 328–334 (1987).
Van Slogteren GMS, Hoge JHC, Hooykaas PJJ, Schilperoort RA: Clonal analysis of heterogeneous crown gall tumor tissues induced by wild-type and shooter mutant strains of Agrobacterium tumefaciens-expression of T-DNA genes. Plant Mol Biol 2: 321–333 (1983).
Verheyden P, Pletinckx J, Maes D, Pepermans HAM, Wyns L, Willem R, Martins JC: 1H NMR study of the interaction of N,N0, N00-triacetyl chitotriose with Ac-AMP2, a sugar binding antimicrobial protein isolated from Amaranthus caudatus. FEBS Lett 370: 245–249 (1995).
Verwoerd TC, Dekker BMM, Hoekema A: A small-scale procedure for the rapid isolation of plant RNAs. Nucl Acids Res 17: 2362 (1989).
Vierheilig H, Iseli B, Alt M, Raikhel N, Wiemken A, Boller T: Resistance of Urtica dioica to mycorrhizal colonization: a possible involvement of Urtica dioica agglutinin. Plant Soil 183: 131–136 (1996).
Vretblad P: Purification of lectins by biospecific affinity chromatography. Biochim Biophys Acta 434: 169–176 (1976).
Walujono K, Scholma RA, Beintema JJ, Mariono A, Hahn AM: Amino acid sequence of hevein. In: Proc Int Rubber Conf, Kuala Lumpur 2: 518–531 (1975).
Wright CS: Structural comparison of the two distinct sugar binding sites in wheat germ agglutinin isolectin 2. J Mol Biol 178: 91–104 (1984).
Wright CS: Refinement of the crystal structure of wheat germ agglutinin isolectin 2 at 1.8Å resolution. J Mol Biol 194: 501–529 (1987).
Wright CS: 2.2Å resolution structure analysis of two refined N-acetylneuraminyl-lactose-wheat germ agglutinin isolectin complexes. J Mol Biol 215: 635–651 (1990). 347
Wright HT, Sandrasegaram G, Wright CS: Evolution of a family of N-acetylglucosamine binding proteins containing the disulfide-rich domain of wheat germ agglutinin. J Mol Evol 33: 283–294 (1991).
Yamaguchi K, Mori A, Funatsu G: Amino acid sequence and some properties of lectin-D from the roots of pokeweed (Phytolacca americana). Biosci Biotech Biochem 60: 1380–1382 (1996).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Does, M.P., Ng, D.K., Dekker, H.L. et al. Characterization of Urtica dioica agglutinin isolectins and the encoding gene family. Plant Mol Biol 39, 335–347 (1999). https://doi.org/10.1023/A:1006134932290
Issue Date:
DOI: https://doi.org/10.1023/A:1006134932290