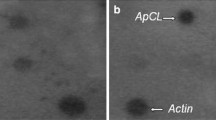
Abstract
Lateral root formation in root cultures of Arabidopsis thaliana can be initiated by exogenous addition of auxin. In order to find cDNA clones of which the corresponding mRNAs accumulate during this process, a cDNA library was constructed from root cultures treated with the active auxin 1-naphthaleneacetic acid (1- NAA). Differential screening of this library with cDNA probes derived from mRNA populations isolated from root cultures treated with 1-NAA and the inactive analogue 2-naphthaleneacetic acid (2-NAA) led to the isolation of four cDNA clones, designated AIR1, AIR3, AIR9 and AIR12. Accumulation of the mRNAs starts between 4 and 8 h and continues till at least 24 h after addition of an active auxin. Sequence analysis revealed that AIR1 encodes a protein that is related to a large family of proteins that consist of a proline-rich or glycine- rich N-terminus and a hydrophobic, possibly membrane spanning C- terminus. The putative function of these proteins is coupling of the cell wall to the plasma membrane. Surprisingly, AIR1 lacks the proline-rich or glycine-rich N-terminus which is thought to be important for interaction with the cell wall. AIR3 encodes a subtilisin-like serine protease which is believed to be active outside the plant cell. Although AIR9 and AIR12 do not show any significant homology to sequences in the database, they are also predicted to function outside the cell. Our screening thus indicates that a variety of genes encoding extracellular proteins are activated during auxin-induced lateral root formation.
Similar content being viewed by others
References
Ahn JH, Choi Y, Kwon YM, Kim SG, Choi YD, Lee JS: A novel extensin gene encoding a hydroxyproline-rich glycoprotein requires sucrose for its wound-inducible expression in transgenic plants. Plant Cell 8: 1477–1490 (1996).
Aleith F, Richter G: Gene expression during induction of somatic embryogenesis in carrot cell suspensions. Planta 183: 17–24 (1990).
Bánfalvi Z, Molnár A, Molnár G, Lakatos L, Szabó L: Starch synthesis-, and tuber storage protein genes are differently expressed in Solanum tuberosum and in Solanum brevidens. FEBS Lett 383: 159–164 (1996).
Barr PJ: Mammalian subtilisins: the long-sought dibasic processing endoproteases. Cell 66: 1–3 (1991).
Baud F, Pebay-Peyroula E, Cohen-Addad C, Odani S, Lehmann MS: Crystal structure of hydrophobic protein from soybean; a member of a new cysteine-rich family. J Mol Biol 231: 877–887 (1993).
Birnboim HC, Doly J: A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucl Acids Res 7: 1513–1523 (1979).
Blakely LM, Blakely RM, Colowit PM, Elliott DS: Experimental studies on lateral root formation in radish seedling roots. II. Analysis of the dose-response to exogenous auxin. Plant Physiol 87: 414–419 (1988).
Callis J, Carpenter T, Sun CW, Vierstra RD: Structure and evolution of genes encoding polyubiquitin and ubiquitin-like proteins in Arabidopsis thaliana ecotype Columbia. Genetics 139: 921–939 (1995).
Cassab GI, Varner JE: Cell wall proteins. Annu Rev Plant Physiol Plant Mol Biol 39: 321–353 (1988).
Castonguay Y, Laberge S, Nadeau P, Vézina LP: A coldinduced gene from Medicago sativa encodes a bimodular protein similar to developmentally regulated proteins. Plant Mol Biol 24: 799–804 (1994).
Celenza JL Jr., Grisafi PL, Fink GR: A pathway for lateral root formation in Arabidopsis thaliana. Genes Dev 9: 2131–2142 (1995).
Choi DW, Song JY, Kwon YM, Kim SG: Characterization of a cDNA encoding a proline-rich 14 kDa protein in developing cortical cells of the roots of bean (Phaseolus vulgaris) seedlings. Plant Mol Biol 30: 973–982 (1996).
Coupe SA, Taylor JE, Isaac PG, Roberts JA: Identification and characterization of a proline-rich mRNA that accumulates during pod development in oilseed rape (Brassica napus L.). Plant Mol Biol 23: 1223–1232 (1993).
Czakó M, Wilson J, Yu X, Márton L: Sustained root culture for generation and vegetative propagation of transgenic Arabidopsis thaliana. Plant Cell Rep 12: 603–606 (1993).
Datta N, LaFayette PR, Kroner PA, Nagao PT, Key JL: Isolation and characterization of three families of auxin downregulated cDNA clones. Plant Mol Biol 21: 859–869 (1993).
Davies PJ: Plant Hormones and their Role in Plant Growth and Development. Kluwer Academic Publishers, Dordrecht, Netherlands (1996).
Deutch CE, Winicov I: Post-transcriptional regulation of a salt-inducible alfalfa gene encoding a putative chimeric proline-rich cell wall protein. Plant Mol Biol 27: 411–418 (1995).
Devereux J, Haeberli P, Smithies O: A comprehensive set of sequence analysis programs for the VAX. Nucl Acids Res 12: 387–395 (1984).
Doerner P, Jørgensen JE, You R, Steppuhn J, Lamb C: Control of root growth and development by cyclin expression. Nature 380: 520–523 (1996).
Dolan L, Janmaat K, Willemsen V, Linstead P, Poethig S, Roberts K, Scheres B: Cellular organization of the Arabidopsis thaliana root. Development 119: 71–84 (1993).
Feinberg AP, Vogelstein B: A technique for radiolabelling restriction endonuclease fragments to high specific activity. Anal Biochem 132: 6–13 (1983).
Ferreira PC, Hemerly AS, Engler JD, Van Montagu M, Engler G, lnzé D: Developmental expression of the Arabidopsis cyclin gene cyclAt. Plant Cell 6: 1763–1774 (1994).
Fujita T, Kouchi H, Takanari I, Sy¯ono K: Cloning of cDNAs for genes that are specifically or preferentially expressed during the development of tobacco genetic tumors. Plant J 5: 645–654 (1997).
Goodwin W, Pallas JA, Jenkins GI: Transcripts of a gene encoding a putative cell wall-plasma membrane linker protein are specifically cold-induced in Brassica napus. Plant Mol Biol 31: 771–781 (1996).
Hotze M, Waitz A, Schröder J: cDNA for a 14-kilodalton polypeptide from Madagascar periwinkle (Catharanthus roseus). Plant Physiol 104: 1097–1098 (1994).
John I, Wang H, Held BM, Syrkin Wurtele E, Colbert JT: An mRNA that specifically accumulates in maize roots delineates a novel subset of developing cortical cells. Plant Mol Biol 20: 821–831 (1992).
Josè-Estanyol M, Ruiz-Avila L, Puigdomènech P: A maize embryo-specific gene encodes a proline-rich and hydrophobic protein. Plant Cell 4: 413–423 (1992).
Joshi CP: Putative polyadenylation signals in nuclear genes of higher plants: a compilation and analysis. Nucl Acids Res 15: 9627–9640 (1987).
Kaneda M, Tominaga N: Isolation and characterization of a protease from the sarcocarp of melon fruit. J Biochem 78: 1287–1296 (1975).
Keller B, Lamb CJ: Specific expression of a novel cell wall hydroxyproline-rich glycoprotein gene in lateral root initiation. Genes Dev 3: 1639–1646 (1989).
Kieliszewski MJ, Lamport DT: Extensin: repetitive motifs, functional sites, post-translational codes, and phylogeny. Plant J 5: 157–172 (1994).
King GA, O'Donoghue EM, Borst WM, Davies KM, Moyle RL, Farnden KJ: Identification and characterization of an mRNA encoding a proline-rich protein that rapidly declines in abundance in the tips of harvested asparagus spears. Plant Cell Physiol 37: 706–710 (1996).
Kobayashi T, Kobayashi E, Sato S, Hotta Y, Miyajima N, Tanaka A, Tabata S: Characterization of cDNAs induced in meiotic prophase in lily microsporocytes. DNA Res 1: 15–26 (1994).
Malamy JE, Benfey PN: Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124: 33–44 (1997).
Martinez MC, Jørgensen JE, Lawton MA, Lamb CJ, Doerner PW: Spatial pattern of cdc2 expression in relation to meristem activity and cell proliferation during plant development. Proc Natl Acad Sci USA 89: 7360–7364 (1992).
McGurl B, Pearce G, Orozco-Cardenas M, Ryan CA: Structure, expression, and antisense inhibition of the systemin precursor gene. Science 255: 1570–1573 (1992).
Murashige T, Skoog F: A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15: 473–497 (1962).
Nielsen H, Engelbrecht J, Brunak S, von Heijne G: Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng 10: 1–6 (1997).
Nishida T, Ohnishi N, Kodama H, Komamine A: Establishment of synchrony by starvation and readdition of auxin in suspension cultures of Catharanthus roseus cells. Plant Cell Tissue Organ Cult 28: 37–43 (1992).
Odani S, Koide T, Ono T, Seto Y, Tanaka T: Soybean hydrophobic protein. Isolation, partial characterization and the complete primary structure. Eur J Biochem 162: 485–491 (1987).
Ribeiro A, Akkermans AD, van Kammen A, Bisseling T, Pawlowski K: A nodule-specific gene encoding a subtilisinlike protease is expressed in early stages of actinorhizal nodule development. Plant Cell 7: 785–794 (1995).
Richards KD, Gardner RC: pEARLI1: an Arabidopsis member of a conserved gene family. Plant Physiol 109: 1497 (1995).
Romano CP, Robson PR, Smith H, Estelle M, Klee H: Transgene-mediated auxin overproduction in Arabidopsis: hypocotyl elongation phenotype and interactions with the hy6–1 hypocotyl elongation and axr1 auxin-resistant mutants. Plant Mol Biol 27: 1071–1083 (1995).
Rudenskaya GN, Bogdanova EA, Revina LP, Golovkin BN, Stepanov VM: Macluralisin a serine proteinase from fruits of Maclura pomifera (Raf.) Schneid. Planta 196: 174–179 (1995).
Ruegger M, Dewey E, Hobbie L, Brown D, Bernasconi P, Turner J, Muday G, Estelle M: Reduced naphthylphthalamic acid binding in the tir3 mutant of Arabidopsis is associated with a reduction in polar auxin transport and diverse morphological defects. Plant Cell 9: 745–757 (1997).
Sabala I, Franzén H, von Arnold S: A spruce gene, af70, constitutively expressed in somatic embryos and induced by ABA and low temperature in seedlings. Physiol Plant 99: 316–322 (1997).
Sakuma Y, Azuma T, Kato Y, Kojima Y, Miura K: Cytokinininduced mRNA in the suspension cultured cell of poplar (Populus nigra). Mokuzai Gakkaishi 42: 789–794 (1996).
Salts Y, Wachs R, Gruissem W, Barg R: Sequence coding for a novel proline-rich protein preferentially expressed in young tomato fruit. Plant Mol Biol 17: 149–150 (1991).
Sambrook L, Fritsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY (1989).
Sanger F, Nicklen S, Coulson AR: DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74: 5463–5467 (1977).
Santino CG, Stanford GL, Conner TW: Developmental and transgenic analysis of two tomato fruit enhanced genes. Plant Mol Biol 33: 405–416 (1997).
Schaller A, Ryan CA: Identification of a 50-kDa systemin binding protein in tomato plasma membranes having Kex2plike properties. Proc Natl Acad Sci USA 91: 11802–11806 (1994).
Schuurink RC: Molecular and ultrastructural studies of the aleurone layer in dormant and non-dormant barley grains. Ph.D. thesis, University of Leiden, Netherlands (1993).
Showalter AM: Structure and function of plant cell wall proteins. Plant Cell 5: 9–23 (1993).
Siezen RJ, de Vos WM, Leunissen JA, Dijkstra BW: Homology modelling and protein engineering strategy of subtilases, the family of subtilisin-like serine proteinases. Protein Eng 4: 719–737 (1991).
Silen JL, Agard DA: The alpha-lytic protease pro-region does not require a physical linkage to activate the protease domain in vivo. Nature 341: 462–464 (1989).
Subramaniam K, Ranie J, Srinivasa BR, Sinha AM, Mahadevan S: Cloning and sequence of a cDNA encoding a novel hybrid proline-rich protein associated with cytokinin-induced haustoria formation in Cuscuta reflexa. Gene 141: 207–210 (1994).
Taylor BH, Scheuring CF: A molecular marker for lateral root initiation: the RSI-1 gene of tomato (Lycopersicon esculentum Mill) is activated in early lateral root primordia. Mol Gen Genet 243: 148–157 (1994).
Taylor BH, Young RJ, Scheuring CF: Induction of a proteinase inhibitor II-class gene by auxin in tomato roots. PlantMol Biol 23: 1005–1014 (1993).
Taylor AA, Horsch A, Rzepczyk A, Hasenkampf CA, Riggs CD: Maturation and secretion of a serine proteinase is associated with events of late microsporogenesis. Plant J 12: 1261–1271 (1997).
Tornero P, Conejero V, Vera P: Primary structure and expression of a pathogen-induced protease (PR-P69) in tomato plants: similarity of functional domains to subtilisin-like endoproteases. Proc Natl Acad Sci USA 93: 6332–6337 (1996).
Tornero P, Conejero V, Vera P: Identification of a new pathogen-induced member of the subtilisin-like processing protease family from plants. J Biol Chem 272: 14412–14419 (1997).
Torrey JG: The induction of lateral roots by indoleacetic acid and root decapitation. Am J Bot 37: 257–264 (1950).
van de Sande K, Pawlowski K, Czaja I, Wieneke U, Schell J, Schmidt J, Walden R, Matvienko M, Wellink J, van Kammen A, Franssen H, Bisseling T: Modification of phytohormone response by a peptide encoded by EN0D40 of legumes and a nonlegume. Science 273: 370–373 (1996).
van der Zaal EJ, Memelink J, Mennes AM, Quint A, Libbenga KR: Auxin-induced mRNA species in tobacco cell cultures. Plant Mol Biol 10: 145–157 (1987).
van Slogteren GMS, Hoge JHC, Hooykaas PJJ, Schilperoort RA: Clonal analysis of heterogenous crown gall tumor tissues induced by wildtype and shooter mutant strains of Agrobacterium tumefaciens: expression of T-DNA genes. Plant Mol Biol 2: 321–333 (1983).
Vera P, Lamb C, Doerner PW: Cell-cycle regulation of hydroxyproline-rich glycoprotein HRGPnt3 gene expression during the initiation of lateral root meristems. Plant J 6: 717–727 (1994).
Winicov I, Deutch CE: Characterization of a cDNA clone from salt-tolerant alfalfa cells that identifies salt-inducible root-specific transcripts. J Plant Physiol 144: 222–228 (1994).
Xu Y, Buchholz WG, DeRose RT, Hall TC: Characterization of a rice gene family encoding root-specific proteins. Plant Mol Biol 27: 237–248 (1995).
Yaish SA: Ph.D thesis, University of Durham (1990).
Yamagata H, Masuzawa T, Nagaoka Y, Ohnishi T, Iwasaki T: Cucumisin, a serine protease from melon fruits, shares structural homology with subtilisin and is generated from a large precursor. J Biol Chem 269: 32725–32731 (1994).
Yasuda E, Ebinuma H, Wabiko H: A novel glycinerich/ hydrophobic 16 kDa polypeptide gene from tobacco: similarity to proline-rich protein genes and its wound-inducible and developmentally regulated expression. Plant Mol Biol 33: 667–678 (1997).
Zhu XL, Ohta Y, Jordan F, Inouye M: Pro-sequence of subtilisin can guide the refolding of denatured subtilisin in an intramolecular process. Nature 339: 483–484 (1989).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Neuteboom, L.W., Ng, J.M., Kuyper, M. et al. Isolation and characterization of cDNA clones corresponding with mRNAs that accumulate during auxin-induced lateral root formation. Plant Mol Biol 39, 273–287 (1999). https://doi.org/10.1023/A:1006104205959
Issue Date:
DOI: https://doi.org/10.1023/A:1006104205959